Functional isomers are compounds with the same molecular formula but different functional groups, resulting in distinct chemical properties and reactivities. Understanding the differences between functional isomers is essential in fields like organic chemistry and pharmaceuticals, where structural variations impact biological activity. Explore the rest of the article to discover how functional isomers influence chemical behavior and practical applications.
Table of Comparison
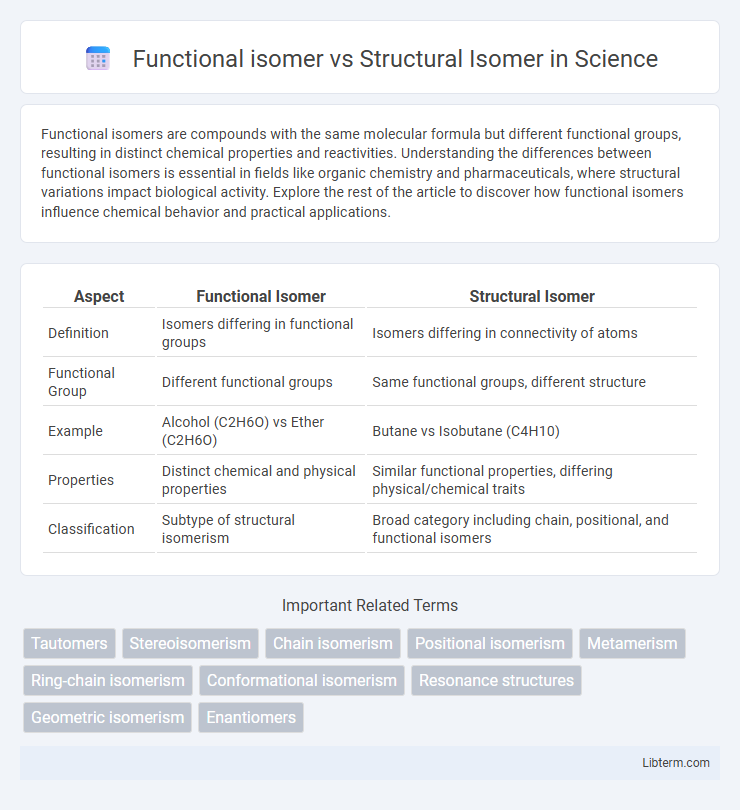
| Aspect | Functional Isomer | Structural Isomer |
|---|---|---|
| Definition | Isomers differing in functional groups | Isomers differing in connectivity of atoms |
| Functional Group | Different functional groups | Same functional groups, different structure |
| Example | Alcohol (C2H6O) vs Ether (C2H6O) | Butane vs Isobutane (C4H10) |
| Properties | Distinct chemical and physical properties | Similar functional properties, differing physical/chemical traits |
| Classification | Subtype of structural isomerism | Broad category including chain, positional, and functional isomers |
Introduction to Isomerism
Isomerism refers to compounds with the same molecular formula but different arrangements of atoms, leading to distinct properties. Functional isomers differ in the functional groups attached to the molecules, causing variations in chemical behavior and reactivity. Structural isomers, also known as constitutional isomers, vary in the connectivity of atoms within the molecule, affecting physical and chemical properties without changing the molecular formula.
Defining Functional Isomers
Functional isomers are compounds that have the same molecular formula but differ in the type of functional group present, resulting in distinct chemical properties. Unlike structural isomers, which vary in the connectivity of atoms without necessarily changing the functional groups, functional isomers exhibit variations in functional groups such as alcohols, ethers, aldehydes, or ketones. The presence of different functional groups in functional isomers leads to diverse reactivity and physical characteristics despite identical molecular formulas.
Understanding Structural Isomers
Structural isomers are compounds with the same molecular formula but different connectivity of atoms, resulting in distinct physical and chemical properties. Unlike functional isomers, which have different functional groups, structural isomers vary in bond arrangements such as chain branching or position of substituents. Understanding structural isomers involves analyzing variations in carbon skeletons, position isomerism, and functional group location without changing the molecular formula.
Key Differences between Functional and Structural Isomers
Functional isomers differ in the functional groups attached to the carbon skeleton, whereas structural isomers vary in the connectivity or arrangement of atoms within the molecule. Functional isomers exhibit distinct chemical properties due to different functional groups, while structural isomers share the same molecular formula but differ in carbon chain branching, position of functional groups, or ring structures. Key differences include variations in chemical reactivity, physical properties, and molecular geometry arising from their unique atomic arrangements.
Types of Functional Isomerism
Functional isomerism occurs when compounds with the same molecular formula contain different functional groups, resulting in distinct chemical properties, such as alcohols and ethers with the formula C2H6O. Structural isomerism, a broader category, includes chain isomerism, position isomerism, and functional group isomerism, with functional isomers specifically differing in their functional groups like aldehydes versus ketones (C3H6O). Recognizing types of functional isomerism is crucial for understanding variations in reactivity and applications among isomeric compounds in organic chemistry.
Types of Structural Isomerism
Functional isomers are compounds with the same molecular formula but different functional groups, resulting in distinct chemical properties. Structural isomers differ in the connectivity of atoms, and types of structural isomerism include chain isomerism (variations in carbon chain length), position isomerism (different positions of functional groups), and functional group isomerism (different functional groups within the same formula). These variations impact physical and chemical behaviors, making the identification of structural isomers essential in organic chemistry.
Real-world Examples of Functional Isomers
Functional isomers are compounds with the same molecular formula but different functional groups, resulting in distinctly different chemical properties, such as ethanol (an alcohol) and dimethyl ether (an ether), both C2H6O. Structural isomers share the same molecular formula but differ in the connectivity of atoms, like butanol and 2-methylpropanol, both C4H10O, demonstrating variations in carbon chain arrangements rather than functional groups. Functional isomer examples like acetone (a ketone) and propanal (an aldehyde), both C3H6O, are crucial in industrial applications due to their differing reactivity and uses in solvents and chemical synthesis.
Common Structural Isomer Examples
Functional isomers differ in the type of functional groups attached to the same molecular formula, whereas structural isomers vary in the connectivity of atoms. Common structural isomer examples include n-butane and isobutane, which both have the formula C4H10 but different carbon chain arrangements. Another example involves C3H6O compounds like acetone and propanal, illustrating isomers with distinct functional groups and structures.
Importance of Isomerism in Chemistry
Functional isomers differ in the functional groups attached to the same molecular formula, while structural isomers vary in the connectivity of atoms within the molecule. The importance of isomerism in chemistry lies in its impact on physical and chemical properties, enabling the design of substances with targeted functions and behaviors. Understanding isomerism aids in drug development, materials science, and the elucidation of biochemical pathways critical for innovation and application.
Applications and Significance of Isomers
Functional isomers, differing in functional groups, play crucial roles in pharmaceuticals where varied biological activities arise from distinct chemical functionalities, enhancing drug design and specificity. Structural isomers, characterized by different atom connectivity, are significant in material science and organic synthesis, enabling the development of compounds with unique physical properties and reactivity profiles. Understanding these isomers aids in optimizing chemical processes, improving product performance, and advancing research in medicinal chemistry and industrial applications.
Functional isomer Infographic
 libterm.com
libterm.com