Compound structures enhance chemical complexity by combining two or more elements or molecules, resulting in unique properties and applications in fields like pharmaceuticals, materials science, and agriculture. Understanding the behavior and interactions of compounds is essential for innovations and practical solutions in various industries. Explore the rest of this article to uncover how compounds impact your daily life and technological advancements.
Table of Comparison
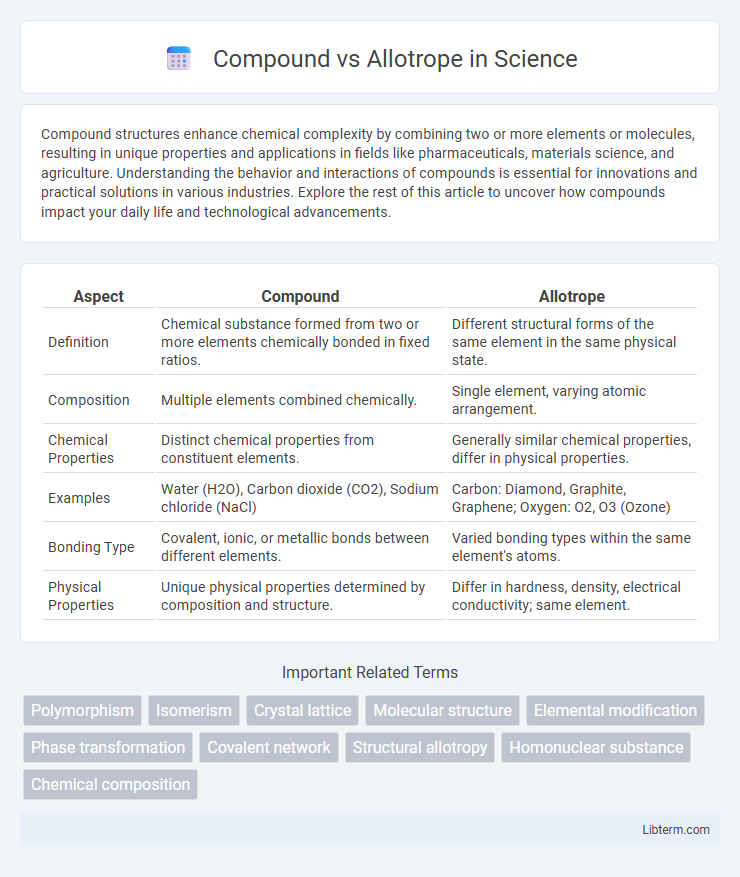
| Aspect | Compound | Allotrope |
|---|---|---|
| Definition | Chemical substance formed from two or more elements chemically bonded in fixed ratios. | Different structural forms of the same element in the same physical state. |
| Composition | Multiple elements combined chemically. | Single element, varying atomic arrangement. |
| Chemical Properties | Distinct chemical properties from constituent elements. | Generally similar chemical properties, differ in physical properties. |
| Examples | Water (H2O), Carbon dioxide (CO2), Sodium chloride (NaCl) | Carbon: Diamond, Graphite, Graphene; Oxygen: O2, O3 (Ozone) |
| Bonding Type | Covalent, ionic, or metallic bonds between different elements. | Varied bonding types within the same element's atoms. |
| Physical Properties | Unique physical properties determined by composition and structure. | Differ in hardness, density, electrical conductivity; same element. |
Introduction to Compounds and Allotropes
Compounds consist of two or more different elements chemically bonded in fixed proportions, forming substances with unique properties distinct from their constituent elements. Allotropes refer to different structural forms of the same element, exhibiting variations in physical and chemical properties due to differences in atomic arrangement. Understanding compounds and allotropes is essential for exploring material characteristics and chemical reactivity in diverse scientific fields.
Definition of Compound
A compound is a chemical substance formed when two or more elements chemically bond in fixed proportions, resulting in new properties distinct from its constituent elements. Unlike allotropes, which are different structural forms of the same element, compounds consist of different elements combined at the molecular or atomic level. Understanding the precise chemical composition of compounds is fundamental to chemistry, as it determines reactivity, behavior, and applications across various industries.
Definition of Allotrope
Allotropes are different structural forms of the same element, where atoms are bonded together in distinct arrangements, resulting in varied physical and chemical properties. Unlike compounds, which consist of two or more different elements chemically combined in fixed ratios, allotropes involve only one element in multiple forms, such as carbon existing as diamond, graphite, and graphene. Understanding allotropy is crucial in fields like materials science and chemistry for exploiting unique properties of elements in different allotrope forms.
Key Differences Between Compounds and Allotropes
Compounds consist of two or more elements chemically bonded in fixed ratios, exhibiting distinct chemical properties, while allotropes are different structural forms of the same element, showing varying physical properties without changing chemical composition. Compounds form through chemical reactions producing new substances, whereas allotropes exist due to different atomic arrangements or bonding within the same element, such as graphite and diamond being allotropes of carbon. The key difference lies in composition and bonding: compounds involve multiple elements, while allotropes involve only one element with variations in molecular structure.
Chemical Structure Comparison
Compounds consist of two or more different elements chemically bonded in fixed proportions, creating molecules with unique chemical properties distinct from their constituent elements. Allotropes are different structural forms of the same element, where atoms bond together in varied arrangements, resulting in distinct physical and chemical characteristics. The fundamental difference lies in compounds having heterogeneous atom types combined through chemical bonds, whereas allotropes involve homogeneous atoms rearranged to form different molecular or crystal structures.
Physical Properties of Compounds vs Allotropes
Compounds exhibit consistent physical properties such as fixed melting and boiling points, defined densities, and uniform crystalline structures due to their specific chemical compositions and bonding patterns. Allotropes of the same element display diverse physical properties like variations in hardness, electrical conductivity, and melting points because of differences in atomic arrangements and bonding types within the element. For example, carbon allotropes like diamond and graphite differ significantly in hardness and conductivity despite sharing the same chemical composition, contrasting the uniform properties found in compounds like water (H2O) or sodium chloride (NaCl).
Real-World Examples of Compounds
Water (H2O) and carbon dioxide (CO2) serve as common examples of compounds, consisting of two or more elements chemically bonded in fixed proportions. Sodium chloride (NaCl), or table salt, is another everyday compound vital for culinary and biological functions. Unlike allotropes, where the same element exists in different structural forms such as graphite and diamond for carbon, compounds have distinct chemical formulas and properties defining their unique applications in industries and nature.
Real-World Examples of Allotropes
Carbon exemplifies allotropes through diamond, graphite, and graphene, each with distinct atomic arrangements and properties; diamond is renowned for its hardness and optical clarity, graphite excels in electrical conductivity and lubrication, while graphene offers exceptional strength and flexibility in electronics. Oxygen exists in allotropes such as O2, the breathable gas, and O3 or ozone, a crucial component in the Earth's stratosphere that absorbs harmful ultraviolet radiation. Sulfur allotropes include rhombic and monoclinic forms, differing in crystal structures and melting points, impacting their industrial applications in vulcanization and pharmaceuticals.
Applications and Uses in Industry
Compounds are extensively used in pharmaceuticals, agriculture, and manufacturing due to their fixed chemical composition and predictable properties, enabling the production of medicines, fertilizers, and plastics. Allotropes, such as graphite and diamond forms of carbon, find specialized industrial applications; graphite is used in lubricants and batteries, while diamond is essential for cutting, grinding, and drilling tools. The distinct physical properties of allotropes allow for versatile uses in technology and materials science, whereas compounds provide consistency in chemical reactions and product synthesis.
Conclusion: Choosing Between Compound and Allotrope
Choosing between a compound and an allotrope depends on their distinct chemical properties and structural variations. Compounds consist of two or more elements chemically bonded in fixed ratios, offering predictable chemical behavior for practical applications, while allotropes are different structural forms of the same element with unique physical and chemical characteristics. Understanding these differences is essential for selecting the appropriate form in fields like materials science, chemistry, and industrial applications.
Compound Infographic
 libterm.com
libterm.com