Extraction is a critical process for obtaining valuable substances from raw materials, ensuring efficiency and purity in various industries such as pharmaceuticals, food, and cosmetics. Understanding the extraction techniques can significantly impact the quality and yield of the final product, making it essential for innovation and sustainability. Explore this article to discover the most effective extraction methods tailored to your specific needs.
Table of Comparison
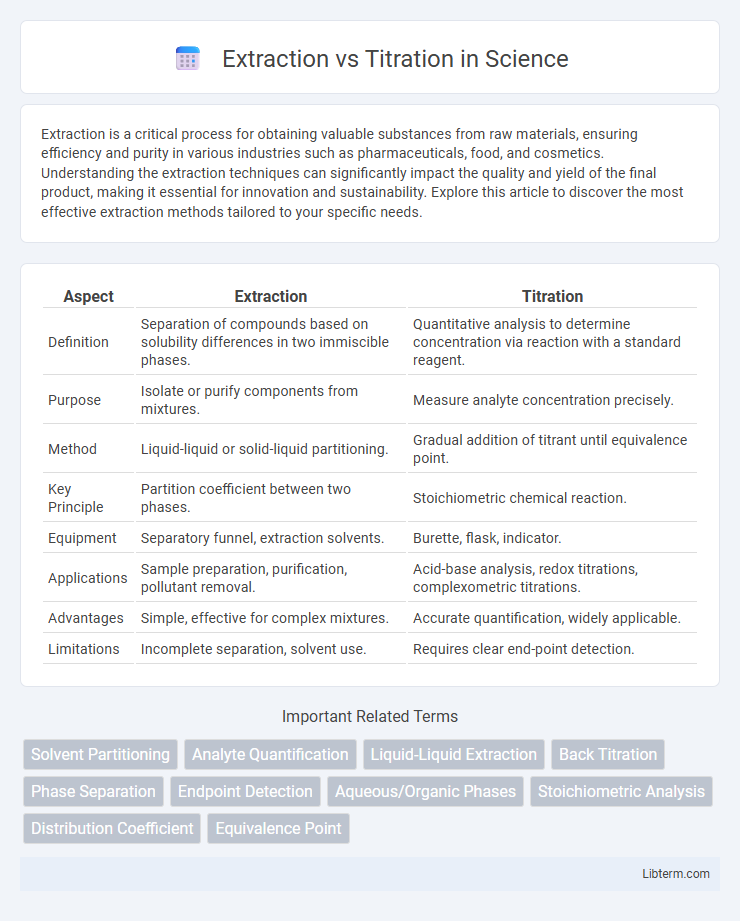
| Aspect | Extraction | Titration |
|---|---|---|
| Definition | Separation of compounds based on solubility differences in two immiscible phases. | Quantitative analysis to determine concentration via reaction with a standard reagent. |
| Purpose | Isolate or purify components from mixtures. | Measure analyte concentration precisely. |
| Method | Liquid-liquid or solid-liquid partitioning. | Gradual addition of titrant until equivalence point. |
| Key Principle | Partition coefficient between two phases. | Stoichiometric chemical reaction. |
| Equipment | Separatory funnel, extraction solvents. | Burette, flask, indicator. |
| Applications | Sample preparation, purification, pollutant removal. | Acid-base analysis, redox titrations, complexometric titrations. |
| Advantages | Simple, effective for complex mixtures. | Accurate quantification, widely applicable. |
| Limitations | Incomplete separation, solvent use. | Requires clear end-point detection. |
Introduction to Extraction and Titration
Extraction separates compounds based on their solubility differences between two immiscible liquids, commonly using solvents to isolate target substances. Titration quantitatively determines analyte concentration through a controlled reaction with a titrant of known concentration, often involving acid-base, redox, or complexometric processes. Both methods are fundamental in analytical chemistry for isolating and quantifying chemical components in mixtures.
Fundamental Principles of Extraction
Extraction relies on the differential solubility of substances between two immiscible phases, typically liquid-liquid systems, to separate components based on their distribution coefficients. Titration involves quantitative chemical reactions to determine the concentration of an analyte by measuring the volume of a titrant required to reach a specific endpoint. Fundamental extraction principles include partition coefficient, phase equilibrium, and selective solubility, which govern the efficiency and selectivity of separating target compounds from mixtures.
Essential Concepts of Titration
Titration is a quantitative analytical technique used to determine the concentration of an unknown solution by reacting it with a standard solution of known concentration, where the precise volume at the equivalence point is measured. It involves key concepts such as the titrant, analyte, equivalence point, endpoint, and indicator, which help identify the completion of the chemical reaction. Unlike extraction, which separates compounds based on solubility differences, titration relies on stoichiometric reactions to calculate molarity or concentration accurately.
Types of Extraction Methods
Liquid-liquid extraction, solid-liquid extraction, and supercritical fluid extraction represent the primary types of extraction methods used to separate compounds based on their solubility differences between two immiscible phases. Each method varies in selectivity, efficiency, and environmental impact, with supercritical fluid extraction offering advanced selectivity and eco-friendly attributes using supercritical CO2 as a solvent. In contrast, titration relies on quantitative chemical reactions to determine analyte concentration, making it more suitable for precise quantification rather than compound separation.
Types of Titration Techniques
Titration techniques include acid-base titration, redox titration, complexometric titration, and precipitation titration, each targeting specific chemical reactions for quantitative analysis. Acid-base titration involves neutralization between acids and bases, while redox titration relies on oxidation-reduction reactions to determine analyte concentration. Complexometric titration uses chelating agents like EDTA for metal ion quantification, and precipitation titration depends on the formation of insoluble compounds to measure analytes.
Key Differences Between Extraction and Titration
Extraction involves separating compounds based on their solubility differences in two immiscible liquids, while titration is a quantitative analytical technique that determines the concentration of a specific substance by reacting it with a titrant of known concentration. Extraction is primarily a physical separation method used to isolate or concentrate components, whereas titration is a chemical method focused on precise measurement and analysis. Key differences include extraction's reliance on phase partitioning and titration's use of stoichiometric reactions to achieve endpoint detection.
Applications of Extraction in Various Industries
Extraction techniques are crucial in the pharmaceutical industry for isolating active compounds from plant materials and complex mixtures. In the food industry, extraction is used to obtain essential oils, flavors, and nutrients to enhance product quality and shelf life. The petrochemical sector relies on extraction to separate valuable hydrocarbons and remove impurities during refining processes.
Applications of Titration in Laboratory Analysis
Titration is widely used in laboratory analysis for determining the concentration of an unknown solution by reacting it with a standard solution of known concentration. Common applications include acid-base titrations to measure pH levels, redox titrations for oxidation-reduction reactions, and complexometric titrations for metal ion quantification. This precise quantitative technique ensures accurate analysis in pharmaceutical, environmental, and food industries.
Advantages and Limitations: Extraction vs Titration
Extraction offers high selectivity for isolating specific compounds from complex mixtures, making it ideal for preparative and analytical purposes; however, it can be time-consuming and requires careful solvent choice to avoid contamination. Titration provides rapid and precise quantification of reactive species with minimal equipment, but it often lacks selectivity in complex matrices and may require prior sample purification. Both methods complement each other, with extraction enhancing sample purity for titration and titration offering quantitative results post-extraction.
Choosing the Right Method: Factors to Consider
Choosing the right analytical method between extraction and titration depends on the sample matrix, target analyte concentration, and required precision. Extraction techniques are preferable for complex mixtures where selective separation of components is critical, while titration is ideal for solutions with well-defined reactive species and when rapid, cost-effective quantification is desired. Factors such as sample volume, chemical compatibility, sensitivity, and available instrumentation also influence the decision-making process.
Extraction Infographic
 libterm.com
libterm.com