Phase refers to a distinct stage within a process or cycle, often marked by specific characteristics or changes in state. Understanding different phases can help you identify critical transitions and optimize outcomes in projects, scientific phenomena, or personal development. Explore the article to learn how recognizing phases enhances your effectiveness across various contexts.
Table of Comparison
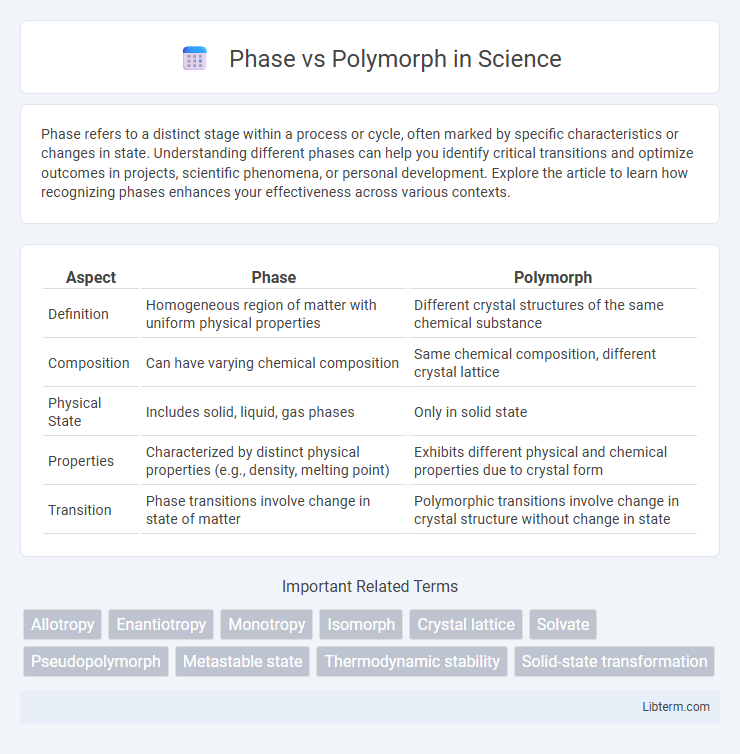
| Aspect | Phase | Polymorph |
|---|---|---|
| Definition | Homogeneous region of matter with uniform physical properties | Different crystal structures of the same chemical substance |
| Composition | Can have varying chemical composition | Same chemical composition, different crystal lattice |
| Physical State | Includes solid, liquid, gas phases | Only in solid state |
| Properties | Characterized by distinct physical properties (e.g., density, melting point) | Exhibits different physical and chemical properties due to crystal form |
| Transition | Phase transitions involve change in state of matter | Polymorphic transitions involve change in crystal structure without change in state |
Introduction to Phases and Polymorphs
Phases refer to distinct physical states of matter characterized by uniform chemical and structural properties, such as solid, liquid, and gas. Polymorphs are specific types of solid phases where a single chemical substance can crystallize into more than one distinct crystal structure. Understanding phases and polymorphs is critical in materials science and pharmaceuticals for controlling properties like solubility, stability, and bioavailability.
Defining Phase in Material Science
Phase in material science refers to a region within a material that has uniform physical and chemical properties, characterized by a distinct atomic arrangement and composition. It is defined by conditions such as temperature, pressure, and composition, resulting in a stable state with homogeneous structure and properties. Polymorphs represent different crystalline phases of the same material, where variations in atomic packing lead to distinct physical attributes despite identical chemical composition.
Understanding Polymorphism
Polymorphism in materials science refers to the ability of a substance to exist in more than one crystal structure or phase, each with distinct physical properties such as melting points and hardness. Understanding polymorphism is crucial for optimizing manufacturing processes and product performance in pharmaceuticals, as different polymorphs of a drug can affect its solubility and bioavailability. Phase, on the other hand, describes the physical state of matter at a given condition, but polymorphism specifically highlights multiple crystalline forms within a single substance.
Key Differences Between Phase and Polymorph
Phase refers to distinct physical states of matter characterized by uniform chemical composition and physical properties, such as solid, liquid, or gas. Polymorph denotes different crystal structures formed by the same chemical substance, influencing material properties like solubility and melting point. The key difference lies in phase describing general state changes, while polymorph focuses on structural variations within the same phase or solid state.
Examples of Phases in Various Materials
Phases in materials represent distinct states of matter characterized by uniform physical and chemical properties within a system, such as solid, liquid, and gas phases of water or the alpha and beta phases in titanium alloys that influence mechanical properties. Examples of phases include the face-centered cubic (FCC) and body-centered cubic (BCC) crystal structures in metals like iron, which determine hardness and ductility, and the liquid crystalline phase in certain organic compounds used in display technologies. Understanding the difference between phases and polymorphs, where polymorphs are different crystal structures of the same chemical compound, is critical for applications in pharmaceuticals, metallurgy, and materials science.
Types of Polymorphism in Chemistry
Polymorphism in chemistry refers to the ability of a compound to crystallize into more than one distinct crystal structure, each known as a polymorph, which can significantly affect the material's physical properties such as melting point, solubility, and stability. Unlike phases, which represent different states of matter (solid, liquid, gas), polymorphs specifically pertain to variations in the crystalline arrangement within the solid phase. Common types of polymorphism include packing polymorphism, conformational polymorphism, and solvomorphism, each defined by differences in molecular packing, molecular conformation, or solvent incorporation respectively.
Importance of Phase Identification
Phase identification is crucial in materials science and chemistry to determine the distinct structural forms of a substance, which directly influence its physical and chemical properties. Differentiating between phases, such as solid, liquid, and gas, ensures accurate understanding of material behavior under varying conditions, while recognizing polymorphs--different crystalline forms of the same compound--helps optimize pharmaceutical efficacy, stability, and manufacturing processes. Advanced techniques like X-ray diffraction (XRD) and differential scanning calorimetry (DSC) are essential for precise phase and polymorph characterization, impacting product quality and performance.
Impact of Polymorphism on Material Properties
Polymorphism significantly influences the mechanical, thermal, and optical properties of materials by enabling multiple crystal structures within the same chemical compound. Different polymorphs exhibit varied atomic arrangements, which affect hardness, melting points, solubility, and electrical conductivity. Understanding these variations is crucial for optimizing material performance in pharmaceuticals, semiconductors, and advanced ceramics.
Methods for Detecting Phases and Polymorphs
X-ray diffraction (XRD) and Raman spectroscopy are primary methods for detecting phases and polymorphs in crystalline materials, providing distinct diffraction patterns and vibrational modes, respectively. Differential scanning calorimetry (DSC) reveals polymorphic transitions through thermal events, while solid-state nuclear magnetic resonance (ssNMR) offers molecular-level insights into structural differences. Advanced techniques, such as synchrotron radiation and electron microscopy, enhance phase and polymorph identification by delivering high-resolution structural and morphological data.
Applications and Implications in Industry
Phase refers to the distinct physical states of a material, such as solid, liquid, or gas, while polymorphs are different crystalline forms of a solid material. Industrial applications of polymorphs are critical in pharmaceuticals, as varying polymorphs can exhibit different solubility and bioavailability, influencing drug efficacy and stability. Understanding phase behavior and polymorphism affects manufacturing processes, material performance, and product quality across sectors like electronics, metallurgy, and chemical production.
Phase Infographic
 libterm.com
libterm.com