Hydrous compounds contain water molecules integrated into their crystal structure, influencing their physical and chemical properties. These materials often play crucial roles in various industries, including pharmaceuticals, geology, and materials science. Discover how understanding hydrous substances can enhance your knowledge and applications by reading the full article.
Table of Comparison
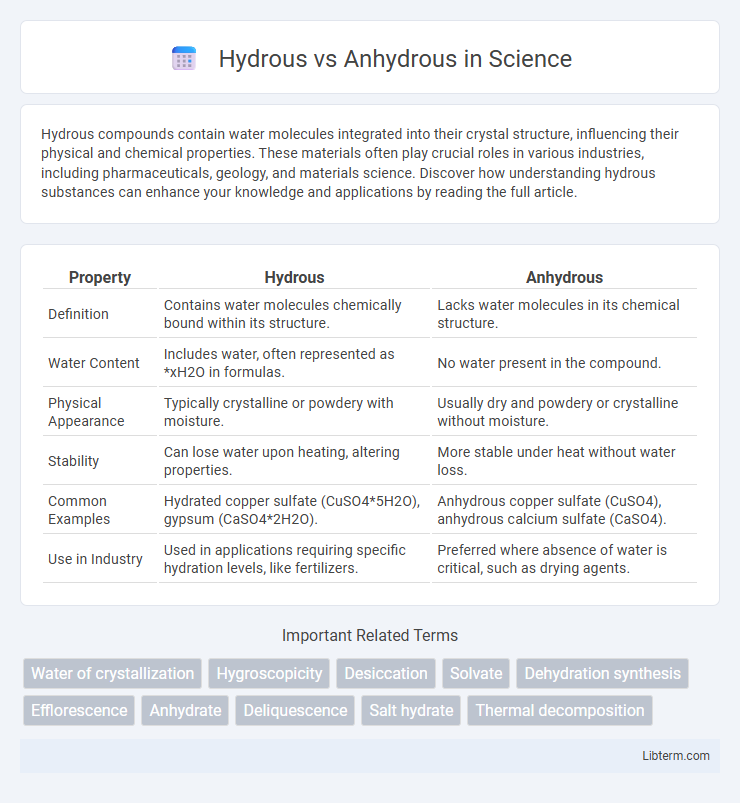
| Property | Hydrous | Anhydrous |
|---|---|---|
| Definition | Contains water molecules chemically bound within its structure. | Lacks water molecules in its chemical structure. |
| Water Content | Includes water, often represented as *xH2O in formulas. | No water present in the compound. |
| Physical Appearance | Typically crystalline or powdery with moisture. | Usually dry and powdery or crystalline without moisture. |
| Stability | Can lose water upon heating, altering properties. | More stable under heat without water loss. |
| Common Examples | Hydrated copper sulfate (CuSO4*5H2O), gypsum (CaSO4*2H2O). | Anhydrous copper sulfate (CuSO4), anhydrous calcium sulfate (CaSO4). |
| Use in Industry | Used in applications requiring specific hydration levels, like fertilizers. | Preferred where absence of water is critical, such as drying agents. |
Introduction to Hydrous and Anhydrous Substances
Hydrous substances contain water molecules integrated into their crystal structure, significantly affecting their physical properties such as solubility and density. Anhydrous substances lack this water content, resulting in different chemical reactivity and stability characteristics. Understanding the distinction between hydrous and anhydrous forms is crucial in industries like pharmaceuticals and chemistry, where hydration state impacts formulation and storage conditions.
Defining Hydrous Compounds
Hydrous compounds contain water molecules integrated into their crystal structure, which significantly influence their physical and chemical properties. These water molecules, known as waters of hydration, often affect the compound's stability, solubility, and weight. Understanding the distinction between hydrous and anhydrous forms is crucial for applications in chemistry, pharmaceuticals, and materials science.
Understanding Anhydrous Compounds
Anhydrous compounds are chemical substances that contain no water molecules within their crystalline structure, distinguishing them from their hydrous counterparts which include water. These compounds are crucial in various industrial applications where moisture can alter chemical reactions, such as in pharmaceuticals, manufacturing, and laboratory settings. Understanding the properties and behavior of anhydrous compounds ensures accurate formulation, storage, and usage, preventing unwanted hydration and maintaining compound stability.
Key Differences Between Hydrous and Anhydrous
Hydrous compounds contain water molecules chemically bound within their crystal structure, whereas anhydrous compounds lack this water content. The presence of water in hydrous substances often impacts their physical properties such as solubility, density, and stability, distinguishing them from their anhydrous counterparts. Industrial applications hinge on these differences, with hydrous forms commonly used in fertilizers and anhydrous types preferred in chemical reactions requiring precise stoichiometry.
Chemical Properties Comparison
Hydrous compounds contain water molecules integrated into their crystal structure, which influences properties such as solubility, melting point, and reactivity. Anhydrous compounds lack these water molecules, typically exhibiting higher thermal stability and different chemical reactivity profiles compared to their hydrous counterparts. The presence or absence of water significantly affects physical properties like density and ionic conductivity, impacting applications across pharmaceuticals, catalysis, and materials science.
Common Examples in Everyday Life
Hydrous compounds contain water molecules bound within their structure, such as copper sulfate pentahydrate (CuSO4*5H2O) commonly found in agriculture and chemistry labs, whereas anhydrous compounds like anhydrous calcium chloride (CaCl2) are used as drying agents in desiccants. In everyday life, hydrated salts appear in products like gypsum (CaSO4*2H2O), essential for drywall manufacturing, while anhydrous forms are prominent in moisture-absorbing packets included in packaging. These distinctions influence their physical properties and practical applications, such as solubility, weight, and storage conditions.
Industrial Applications of Hydrous and Anhydrous Forms
Hydrous compounds, containing water molecules, are extensively used in industries like construction for gypsum plaster and in agriculture as hydrated fertilizers that provide essential moisture to crops. Anhydrous forms, which lack water, are preferred in chemical manufacturing and pharmaceuticals due to their higher purity and stability, aiding precise reactions and prolonged shelf life. Both forms play critical roles in industrial applications where moisture content impacts material properties and performance.
Detection and Identification Methods
Hydrous and anhydrous compounds can be distinguished through spectroscopic methods such as infrared (IR) spectroscopy, where hydrous forms exhibit characteristic O-H stretching vibrations absent in anhydrous types. Thermogravimetric analysis (TGA) provides quantitative data by measuring weight loss corresponding to water release upon heating, enabling precise detection of hydration levels. X-ray diffraction (XRD) techniques further aid identification by revealing structural differences between hydrated and anhydrous crystal lattices.
Storage and Handling Considerations
Hydrous compounds contain water molecules within their structure, making them more sensitive to humidity and requiring storage in airtight, moisture-resistant containers to prevent degradation. Anhydrous substances lack bound water, allowing easier handling but necessitate protection from moisture to avoid hydration and potential chemical changes. Both forms demand clearly labeled storage conditions, with temperature and humidity controls tailored to maintain their chemical stability and safety.
Conclusion: Choosing Between Hydrous and Anhydrous
Choosing between hydrous and anhydrous forms depends on specific application requirements such as moisture content, stability, and reactivity. Hydrous compounds contain water molecules that can enhance solubility and ease of handling, whereas anhydrous forms offer higher purity and longer shelf life ideal for precise chemical reactions. Understanding the role of water in the compound ensures optimal performance and cost-effectiveness in industrial, pharmaceutical, or laboratory settings.
Hydrous Infographic
 libterm.com
libterm.com