Hydrolytic processes play a crucial role in breaking down complex molecules through the addition of water, facilitating numerous biological and chemical reactions. Understanding hydrolytic mechanisms can enhance your knowledge of how substances are decomposed and transformed in natural and industrial environments. Explore the full article to discover the impact and applications of hydrolytic reactions in various fields.
Table of Comparison
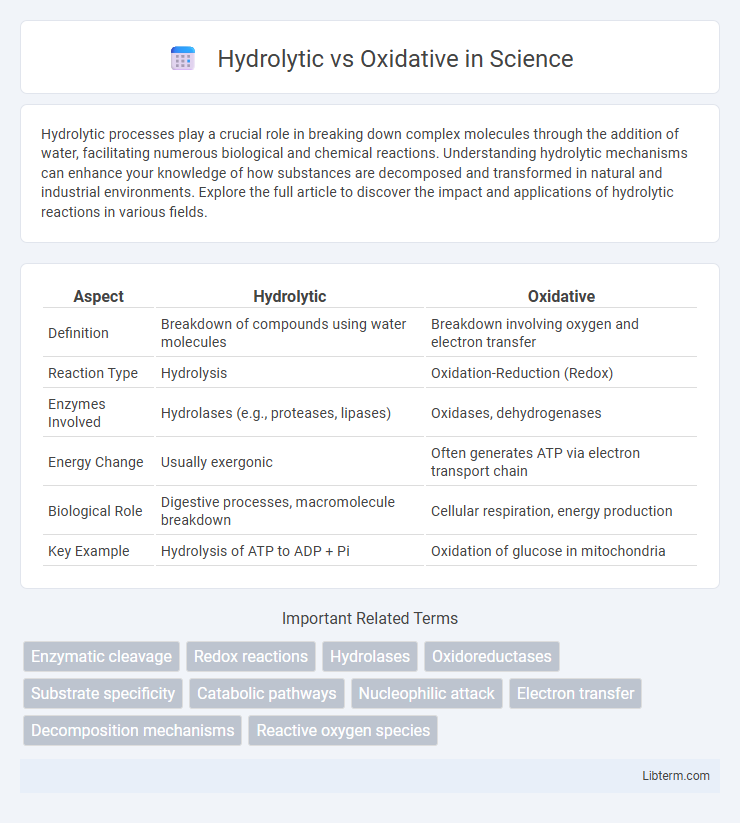
| Aspect | Hydrolytic | Oxidative |
|---|---|---|
| Definition | Breakdown of compounds using water molecules | Breakdown involving oxygen and electron transfer |
| Reaction Type | Hydrolysis | Oxidation-Reduction (Redox) |
| Enzymes Involved | Hydrolases (e.g., proteases, lipases) | Oxidases, dehydrogenases |
| Energy Change | Usually exergonic | Often generates ATP via electron transport chain |
| Biological Role | Digestive processes, macromolecule breakdown | Cellular respiration, energy production |
| Key Example | Hydrolysis of ATP to ADP + Pi | Oxidation of glucose in mitochondria |
Introduction to Hydrolytic and Oxidative Processes
Hydrolytic processes involve the chemical breakdown of compounds through reaction with water, typically catalyzed by enzymes such as hydrolases, which play a crucial role in digestion and metabolism. Oxidative processes rely on the transfer of electrons, often involving oxygen or other oxidizing agents, to convert substances into more oxidized forms, fundamental to cellular respiration and energy production. Both hydrolytic and oxidative mechanisms are essential biochemical pathways that regulate metabolism, detoxification, and energy transformation in living organisms.
Defining Hydrolytic Reactions
Hydrolytic reactions involve the cleavage of chemical bonds through the addition of water molecules, typically breaking esters, amides, or glycosidic linkages. These reactions are catalyzed by hydrolytic enzymes such as proteases, lipases, and nucleases that facilitate substrate breakdown in biological systems. In contrast, oxidative reactions involve electron transfer and the addition of oxygen or removal of hydrogen, playing crucial roles in metabolic pathways like cellular respiration and detoxification.
Understanding Oxidative Reactions
Oxidative reactions involve the transfer of electrons, typically characterized by the addition of oxygen or the removal of hydrogen, which leads to the alteration of molecular structures in biochemical and industrial processes. Enzymes like oxidases and dehydrogenases facilitate these reactions, playing crucial roles in cellular respiration, metabolism, and detoxification pathways. Understanding oxidative mechanisms is essential for advancements in energy production, pharmaceutical synthesis, and environmental technology.
Key Mechanisms: Hydrolysis vs Oxidation
Hydrolytic mechanisms involve the cleavage of chemical bonds through the addition of water molecules, resulting in the breakdown of complex compounds into simpler molecules. Oxidative mechanisms rely on electron transfer processes where substrates are oxidized, often mediated by enzymes such as oxidases or peroxidases, leading to structural changes via oxygen incorporation or removal of electrons. Understanding these distinct pathways is crucial in biochemical reactions, drug metabolism, and environmental degradation studies.
Biological Importance of Hydrolytic Processes
Hydrolytic processes play a crucial role in biological systems by breaking down complex molecules such as proteins, lipids, and carbohydrates into their monomers through the addition of water, facilitating nutrient absorption and cellular metabolism. Enzymes like proteases, lipases, and amylases catalyze hydrolytic reactions, essential for digestion, signal transduction, and waste removal. These processes maintain cellular homeostasis, support energy production, and regulate biochemical pathways, highlighting their indispensable function in living organisms.
Roles of Oxidative Reactions in Living Systems
Oxidative reactions in living systems play a crucial role in energy production by driving cellular respiration through the electron transport chain, where oxygen acts as the final electron acceptor to generate ATP. These reactions facilitate the metabolism of nutrients, enabling the breakdown of glucose and fatty acids into usable energy forms, while also producing reactive oxygen species that participate in cell signaling and immune responses. Unlike hydrolytic reactions that break chemical bonds using water, oxidative processes involve electron transfer and are essential for maintaining cellular homeostasis and supporting metabolic functions.
Industrial Applications: Hydrolytic vs Oxidative Methods
Hydrolytic methods are widely used in industrial applications for breaking down complex polymers like cellulose into simpler sugars through water-mediated cleavage, essential in biofuel production and paper manufacturing. Oxidative methods involve the use of oxidizing agents to modify or degrade organic compounds, playing a critical role in wastewater treatment, chemical synthesis, and environmental remediation by facilitating pollutant breakdown. Industries prioritize hydrolytic processes for enzymatic specificity and mild conditions, while oxidative methods are favored for their efficiency in removing contaminants and transforming chemical structures rapidly.
Environmental Impacts of Hydrolytic and Oxidative Processes
Hydrolytic processes primarily impact the environment by generating wastewater containing hydrolysis byproducts that can lead to increased chemical oxygen demand (COD) and potential toxicity in aquatic systems. Oxidative processes, such as advanced oxidation, produce reactive oxygen species that enhance pollutant degradation but may result in secondary pollutants or increased energy consumption. Both hydrolytic and oxidative treatments influence environmental sustainability through waste management challenges and resource utilization, requiring optimized control to minimize ecological footprints.
Advantages and Limitations: A Comparative Analysis
Hydrolytic processes offer high specificity and operate under mild conditions, making them energy-efficient and suitable for sensitive substrates, but they often require catalysts that may be costly or unstable. Oxidative methods provide rapid reaction rates and can achieve complete oxidation, beneficial for waste degradation and chemical synthesis, yet they may produce harmful byproducts and demand stringent control of reaction parameters. Balancing the environmental impact and operational costs is crucial when selecting between hydrolytic and oxidative pathways for industrial applications.
Future Perspectives and Research Directions
Future research in hydrolytic and oxidative bioprocesses aims to enhance enzyme engineering for improved catalytic efficiency and substrate specificity, enabling sustainable industrial applications. Advances in systems biology and computational modeling are expected to optimize metabolic pathways, facilitating the development of novel biocatalysts with higher tolerance to harsh conditions. Emerging trends also include integrating hydrolytic and oxidative mechanisms in hybrid systems to maximize biomass conversion and reduce environmental impact.
Hydrolytic Infographic
 libterm.com
libterm.com