Gamma decay releases high-energy photons from an excited atomic nucleus, transforming it into a lower energy state without changing its composition. This process plays a crucial role in the stability of radioactive materials and the emission of penetrating radiation. Explore the article to understand how gamma decay influences nuclear physics and its practical applications in medicine and industry.
Table of Comparison
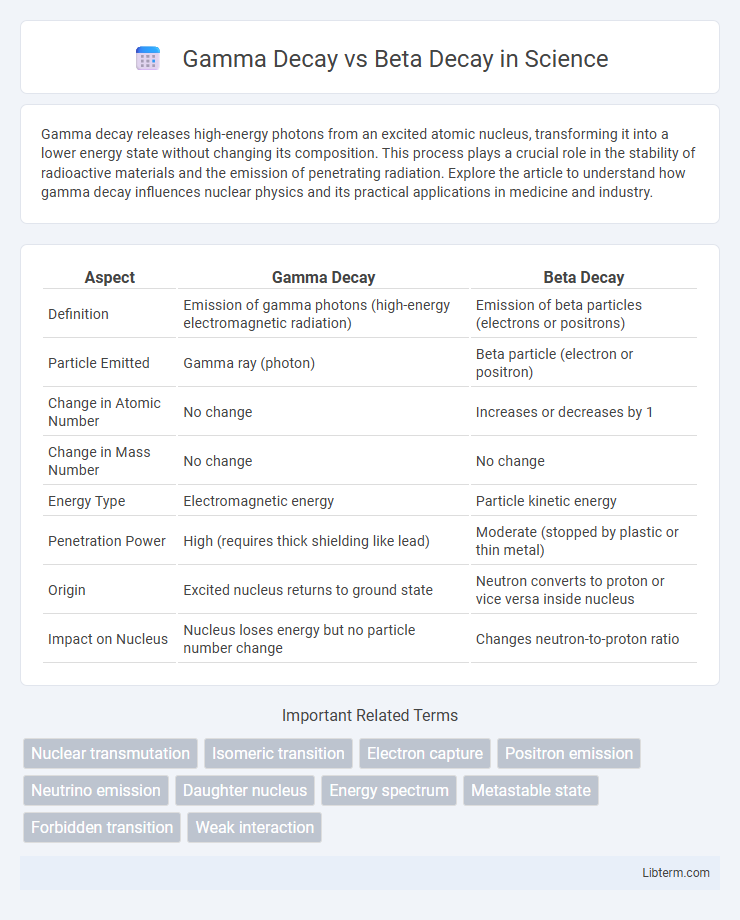
| Aspect | Gamma Decay | Beta Decay |
|---|---|---|
| Definition | Emission of gamma photons (high-energy electromagnetic radiation) | Emission of beta particles (electrons or positrons) |
| Particle Emitted | Gamma ray (photon) | Beta particle (electron or positron) |
| Change in Atomic Number | No change | Increases or decreases by 1 |
| Change in Mass Number | No change | No change |
| Energy Type | Electromagnetic energy | Particle kinetic energy |
| Penetration Power | High (requires thick shielding like lead) | Moderate (stopped by plastic or thin metal) |
| Origin | Excited nucleus returns to ground state | Neutron converts to proton or vice versa inside nucleus |
| Impact on Nucleus | Nucleus loses energy but no particle number change | Changes neutron-to-proton ratio |
Introduction to Nuclear Decay Processes
Gamma decay involves the emission of high-energy photons from an excited atomic nucleus, resulting in the nucleus transitioning to a lower energy state without changing its atomic number or mass. Beta decay consists of the transformation of a neutron into a proton or vice versa within the nucleus, accompanied by the emission of beta particles (electrons or positrons) and neutrinos, altering the atomic number while maintaining the mass number. Both processes are fundamental nuclear decay mechanisms that release energy and contribute to the stability of radioactive isotopes.
What is Gamma Decay?
Gamma decay is a nuclear decay process where an excited nucleus releases excess energy by emitting high-energy photons called gamma rays without changing its atomic number or mass number. Unlike beta decay, which involves the transformation of a neutron into a proton or vice versa with the emission of beta particles (electrons or positrons), gamma decay only reduces the nucleus's energy state. Gamma rays have no mass or charge, making gamma decay a type of electromagnetic radiation emission crucial in nuclear physics and medical imaging.
What is Beta Decay?
Beta decay is a radioactive process in which a neutron in an unstable nucleus transforms into a proton, emitting a beta particle (electron or positron) and an antineutrino or neutrino. This type of decay changes the atomic number of the element, resulting in the transmutation into a different element. Beta decay contrasts with gamma decay, which involves the emission of gamma rays without changing the atomic number or mass of the nucleus.
Key Differences Between Gamma and Beta Decay
Gamma decay releases high-energy photons without changing the atomic number or mass number of the nucleus, resulting in no transmutation of elements. Beta decay involves the transformation of a neutron into a proton (beta-minus) or a proton into a neutron (beta-plus), leading to a change in the atomic number by one while the mass number remains constant. Gamma decay typically follows alpha or beta decay to rid the nucleus of excess energy, whereas beta decay directly alters the nuclear composition by emitting electrons or positrons.
Mechanisms of Gamma vs. Beta Decay
Gamma decay involves the emission of high-energy photons from an excited atomic nucleus, allowing it to transition to a lower energy state without changing the number of protons or neutrons. Beta decay occurs through the transformation of a neutron into a proton or vice versa within the nucleus, accompanied by the emission of a beta particle (electron or positron) and an antineutrino or neutrino, altering the atomic number. The fundamental mechanism of gamma decay is electromagnetic radiation release, while beta decay involves weak nuclear force interactions and particle transmutation.
Types of Radiation Emitted
Gamma decay emits high-energy electromagnetic radiation known as gamma rays, which have no mass or charge and penetrate materials deeply. Beta decay produces beta particles, which are high-speed electrons or positrons with a charge and a small mass, capable of moderate penetration and ionization. Both types of radiation differ in penetration power and ionizing ability, with gamma rays being more penetrating and beta particles causing localized ionization.
Impact on Atomic Structure
Gamma decay alters the atomic nucleus by releasing high-energy photons, leaving the atomic number and mass number unchanged but shifting the nucleus to a lower energy state. Beta decay transforms a neutron into a proton or vice versa, changing the atomic number and thus converting the element into a different isotope or element. The structural changes in beta decay directly affect the chemical properties, whereas gamma decay primarily influences nuclear energy states without modifying the elemental identity.
Energy Release Comparison
Gamma decay releases energy primarily in the form of high-energy photons with no change in atomic number or mass number, typically involving energy levels of several hundred keV to a few MeV. Beta decay emits beta particles (electrons or positrons) along with antineutrinos or neutrinos, resulting in a nuclear transmutation and releasing energy up to a few MeV, often higher than the discrete gamma emissions. The total energy released in beta decay varies depending on the specific isotope and the decay endpoint energy, generally exceeding the photon energy released in gamma decay by enabling mass-to-energy conversion in nuclear transformations.
Applications and Significance in Science
Gamma decay is crucial in medical imaging and cancer radiotherapy due to its high penetration power and ability to sterilize medical equipment. Beta decay plays a significant role in radiocarbon dating, allowing scientists to determine the age of archaeological samples by measuring carbon-14 decay. Both decay types are fundamental in nuclear physics research, enhancing our understanding of atomic stability and nuclear reactions.
Summary: Choosing Between Gamma and Beta Decay
Gamma decay involves the emission of high-energy photons without changing the atomic number or mass number, making it ideal for releasing excess nuclear energy without altering the element. Beta decay transforms a neutron into a proton (beta-minus) or a proton into a neutron (beta-plus), changing the atomic number and thus transmuting the element. Selecting between gamma and beta decay depends on whether a nucleus needs to lose energy while remaining the same element (gamma) or undergo a change in its proton-neutron ratio to achieve stability (beta).
Gamma Decay Infographic
 libterm.com
libterm.com