Antagonism refers to active opposition or hostility between individuals or groups, often resulting in conflict or tension. Understanding the underlying causes and effects of antagonism can help manage disputes and improve relationships in personal and professional settings. Explore this article to learn effective strategies for recognizing and addressing antagonism in your life.
Table of Comparison
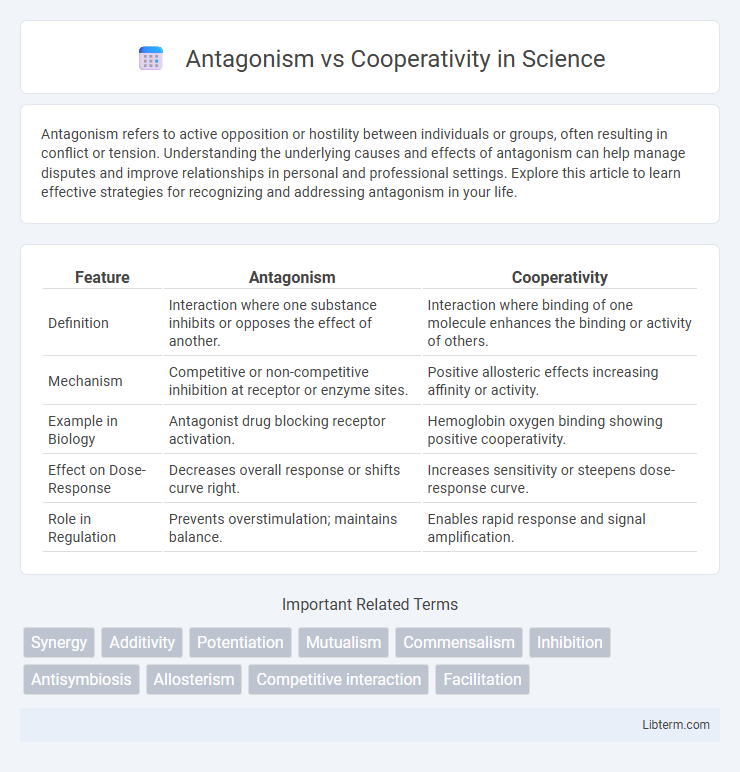
| Feature | Antagonism | Cooperativity |
|---|---|---|
| Definition | Interaction where one substance inhibits or opposes the effect of another. | Interaction where binding of one molecule enhances the binding or activity of others. |
| Mechanism | Competitive or non-competitive inhibition at receptor or enzyme sites. | Positive allosteric effects increasing affinity or activity. |
| Example in Biology | Antagonist drug blocking receptor activation. | Hemoglobin oxygen binding showing positive cooperativity. |
| Effect on Dose-Response | Decreases overall response or shifts curve right. | Increases sensitivity or steepens dose-response curve. |
| Role in Regulation | Prevents overstimulation; maintains balance. | Enables rapid response and signal amplification. |
Understanding Antagonism and Cooperativity
Understanding antagonism involves recognizing how two or more substances or actions produce a combined effect less than the sum of their individual effects, often by inhibiting each other's activity. Cooperativity refers to a scenario where the binding of one molecule influences the binding affinity of additional molecules, enhancing the overall response, commonly observed in allosteric enzymes and hemoglobin oxygen binding. Both concepts play crucial roles in biochemical pathways and drug interactions, impacting therapeutic efficacy and cellular regulation.
Key Differences Between Antagonism and Cooperativity
Antagonism occurs when one molecule inhibits or blocks the effect of another molecule at a receptor site, reducing the overall response, whereas cooperativity refers to the interaction between multiple binding sites on a protein or enzyme where the binding of one ligand influences the binding affinity of additional ligands. In antagonism, the primary outcome is a decrease in activity or response, often seen in competitive or non-competitive receptor interactions, while cooperativity can be positive or negative, modulating the protein's functional activity. Key differences lie in the nature of interaction--antagonism involves inhibitory competition, and cooperativity involves allosteric modulation that alters binding dynamics and function.
The Biological Basis of Antagonism
Antagonism in biological systems occurs when one molecule or ion inhibits or interferes with the action of another, often by binding to the same receptor site without activating it, effectively blocking the natural ligand. This phenomenon is frequently observed in pharmacology where antagonists prevent agonist molecules from triggering a response, providing a mechanism for regulating cellular functions and signal transduction pathways. The molecular basis of antagonism involves competitive or non-competitive inhibition, with competitive antagonists directly competing for receptor sites and non-competitive antagonists binding to alternative sites causing conformational changes that reduce receptor activity.
Mechanisms Driving Cooperativity
Cooperativity in biological systems arises when the binding of a ligand to one site on a multi-subunit protein affects the binding affinity of additional ligand molecules at other sites, often through conformational changes that enhance overall activity. Mechanisms driving cooperativity include allosteric modulation, where ligand binding induces structural shifts that stabilize active conformations, and positive feedback loops that amplify binding events. Such interactions contrast with antagonism, where ligand binding inhibits or reduces activity, highlighting the dynamic regulation of protein function in cellular signaling and metabolic pathways.
Antagonism in Molecular Interactions
Antagonism in molecular interactions occurs when one molecule inhibits or diminishes the effect of another by binding to the same receptor or a different site, preventing activation or signaling. This mechanism is critical in drug design, where antagonists block receptor activity to modulate physiological responses, such as beta-blockers inhibiting adrenaline effects on beta-adrenergic receptors. Understanding molecular antagonism enables targeted therapies and influences pharmacodynamics by altering ligand-receptor binding dynamics.
Cooperativity in Enzyme Function
Cooperativity in enzyme function refers to the phenomenon where the binding of a substrate to one active site increases the affinity of other active sites for the substrate, often observed in multimeric enzymes like hemoglobin. This positive cooperativity enhances enzymatic efficiency and regulation, allowing enzymes to respond dynamically to changes in substrate concentration. Unlike antagonism, which involves inhibitory interactions reducing enzyme activity, cooperativity promotes synergistic effects that optimize metabolic pathways.
Impact on Drug Design and Pharmacology
Antagonism and cooperativity significantly influence drug efficacy and receptor targeting strategies in pharmacology. Antagonism involves one drug inhibiting the effect of another, guiding the design of competitive inhibitors to modulate receptor activity precisely. Cooperativity, where ligand binding at one site affects binding at another, informs the development of allosteric modulators enhancing or diminishing receptor responses for improved therapeutic outcomes.
Real-World Examples of Antagonism
In pharmacology, antagonism occurs when one drug reduces or blocks the effect of another, as seen with naloxone reversing opioid overdoses by binding to opioid receptors without activating them. Another example includes beta-blockers, which antagonize adrenaline's effects, reducing heart rate and blood pressure in conditions like hypertension. Pesticide interactions demonstrate antagonism too; for instance, some insecticides decrease the effectiveness of herbicides when applied together, highlighting the importance of understanding drug or chemical combinations in real-world applications.
Case Studies Highlighting Cooperativity
Case studies on cooperativity in enzyme kinetics reveal how multiple binding sites interact positively to enhance substrate affinity, as seen in hemoglobin's oxygen-binding behavior. Research on allosteric enzymes like aspartate transcarbamoylase demonstrates cooperative substrate binding that regulates metabolic pathways efficiently. These examples emphasize cooperativity's critical role in modulating biological activity compared to antagonism's inhibitory interactions.
Balancing Antagonism and Cooperativity in Complex Systems
Balancing antagonism and cooperativity in complex systems is essential for maintaining stability and adaptability, as antagonistic interactions regulate competition while cooperative behaviors enhance collective function. Effective modulation between these dynamics ensures robust system performance, preventing dominance of one interaction type that could lead to dysfunction or collapse. Understanding molecular pathways and network structures involved in this balance enables targeted interventions to optimize system resilience and efficiency.
Antagonism Infographic
 libterm.com
libterm.com