Ferromagnetic materials exhibit strong magnetic properties due to the alignment of their atomic magnetic moments in the same direction, creating a permanent magnetization. These materials are critical in various applications, including data storage, electric motors, and transformers, because of their ability to retain magnetic properties even after an external magnetic field is removed. Explore the rest of the article to understand how ferromagnetism influences technology and your daily life.
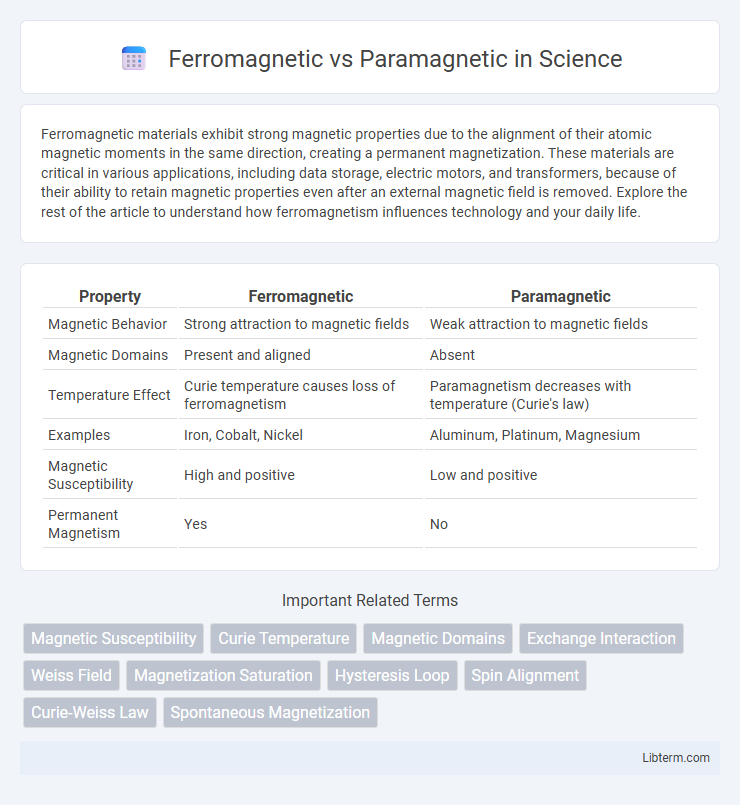
Table of Comparison
| Property | Ferromagnetic | Paramagnetic |
|---|---|---|
| Magnetic Behavior | Strong attraction to magnetic fields | Weak attraction to magnetic fields |
| Magnetic Domains | Present and aligned | Absent |
| Temperature Effect | Curie temperature causes loss of ferromagnetism | Paramagnetism decreases with temperature (Curie's law) |
| Examples | Iron, Cobalt, Nickel | Aluminum, Platinum, Magnesium |
| Magnetic Susceptibility | High and positive | Low and positive |
| Permanent Magnetism | Yes | No |
Introduction to Magnetic Properties
Ferromagnetic materials, such as iron, cobalt, and nickel, exhibit strong magnetic properties due to the alignment of their atomic magnetic moments in the same direction, resulting in spontaneous magnetization even without an external magnetic field. Paramagnetic materials, including aluminum and platinum, show weaker magnetic effects caused by unpaired electrons that align with an external magnetic field but do not retain magnetization once the field is removed. The distinction between ferromagnetism and paramagnetism lies in their magnetic susceptibility and the presence or absence of cooperative atomic magnetic moment alignment.
What is Ferromagnetism?
Ferromagnetism is a magnetic phenomenon where certain materials, such as iron, cobalt, and nickel, exhibit strong, permanent magnetization due to the parallel alignment of their atomic magnetic moments. This alignment results from exchange interactions at the quantum level, creating regions called magnetic domains with uniform magnetization. Ferromagnetic materials retain their magnetization even after the external magnetic field is removed, differentiating them from paramagnetic materials that only exhibit magnetism in the presence of an external field.
What is Paramagnetism?
Paramagnetism is a form of magnetism occurring in materials with unpaired electrons that align their magnetic moments with an external magnetic field, enhancing the overall magnetic effect. Unlike ferromagnetic materials, paramagnetic substances do not retain magnetization after the removal of the external field due to the absence of spontaneous magnetic ordering. Common examples of paramagnetic materials include aluminum, platinum, and oxygen, which exhibit weak magnetic susceptibility influenced by thermal agitation.
Key Differences Between Ferromagnetic and Paramagnetic Materials
Ferromagnetic materials exhibit strong magnetic ordering due to unpaired electron spins aligning parallel within domains, resulting in permanent magnetization even without an external magnetic field. In contrast, paramagnetic materials contain unpaired electrons that align with an external magnetic field only momentarily, producing weak and temporary magnetization that disappears once the field is removed. Ferromagnets, such as iron, cobalt, and nickel, have high magnetic susceptibility and exhibit hysteresis, whereas paramagnets like aluminum and oxygen have low magnetic susceptibility and show no hysteresis.
Atomic Structure and Magnetic Domains
Ferromagnetic materials have highly ordered atomic structures where unpaired electron spins align parallel within regions called magnetic domains, resulting in strong, permanent magnetization. Paramagnetic materials contain unpaired electrons but lack magnetic domains; their atomic spins align only weakly and temporarily with external magnetic fields due to thermal agitation. The presence of magnetic domains in ferromagnetism enables spontaneous magnetization, whereas paramagnetism exhibits magnetization solely under external field influence.
Common Examples of Ferromagnetic and Paramagnetic Substances
Ferromagnetic substances include iron, cobalt, nickel, and their alloys, known for their strong, permanent magnetic properties due to aligned magnetic domains. Paramagnetic substances, such as aluminum, platinum, and manganese, exhibit weak attraction to magnetic fields caused by unpaired electrons but do not retain magnetization once the external field is removed. These distinctions make ferromagnetic materials ideal for permanent magnets and data storage, while paramagnetic materials are commonly used in magnetic resonance imaging and scientific instrumentation.
Magnetic Behavior Under External Fields
Ferromagnetic materials exhibit strong magnetic behavior by aligning their magnetic domains parallel to an external magnetic field, resulting in permanent magnetization even after the field is removed. Paramagnetic materials display weaker magnetization, with unpaired electron spins aligning partially and temporarily along the external magnetic field, causing magnetization only while the field is present. The difference in magnetic susceptibility between ferromagnetic and paramagnetic substances highlights their distinct responses to applied magnetic fields.
Temperature Effects: Curie and Paramagnetic Temperatures
Ferromagnetic materials maintain spontaneous magnetization below the Curie temperature, a critical point where thermal energy disrupts magnetic ordering, causing a phase transition to paramagnetism. Paramagnetic materials exhibit magnetic moments that align with an external magnetic field, but thermal agitation defined by paramagnetic temperature limits their magnetic susceptibility. The Curie temperature specifically delineates the loss of ferromagnetic order, while paramagnetic behavior persists and diminishes gradually with increasing temperature.
Applications of Ferromagnetic and Paramagnetic Materials
Ferromagnetic materials, such as iron, cobalt, and nickel, are extensively used in permanent magnets, magnetic storage devices, and electric motors due to their strong magnetic retention and high coercivity. Paramagnetic materials, including aluminum and platinum, find applications in magnetic resonance imaging (MRI) and as catalysts in chemical reactions because of their weak, positive magnetic susceptibility and quick response to external magnetic fields. The contrasting magnetic behaviors of these materials enable diverse technological applications ranging from data storage to medical diagnostics.
Summary Table: Ferromagnetic vs Paramagnetic Comparison
Ferromagnetic materials exhibit strong magnetic ordering with permanent magnetic moments due to parallel alignment of spins, resulting in high magnetic susceptibility and spontaneous magnetization. Paramagnetic materials have unpaired electrons that align with external magnetic fields but lack spontaneous magnetization, displaying weak and positive magnetic susceptibility that diminishes with increasing temperature. Key distinctions include Curie temperature presence in ferromagnets, magnetic domain formations, and stronger attraction to magnetic fields compared to paramagnets.
Ferromagnetic Infographic
 libterm.com
libterm.com