Oxidation state indicates the degree of oxidation of an atom in a chemical compound, reflecting the number of electrons lost, gained, or shared during a reaction. Understanding oxidation states helps predict compound behavior in redox reactions and is crucial for balancing chemical equations. Explore the article to deepen your knowledge of oxidation states and their practical applications.
Table of Comparison
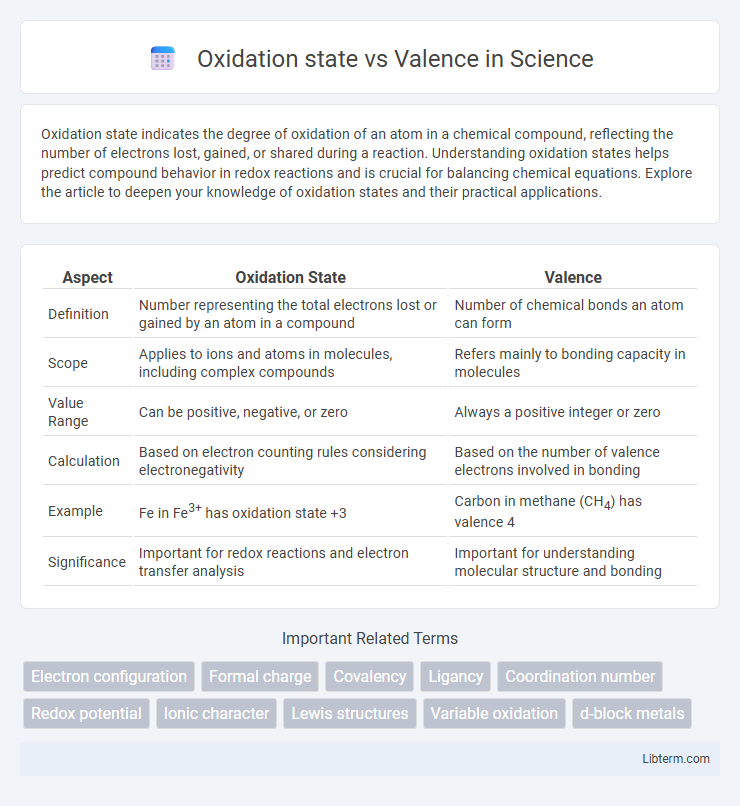
| Aspect | Oxidation State | Valence |
|---|---|---|
| Definition | Number representing the total electrons lost or gained by an atom in a compound | Number of chemical bonds an atom can form |
| Scope | Applies to ions and atoms in molecules, including complex compounds | Refers mainly to bonding capacity in molecules |
| Value Range | Can be positive, negative, or zero | Always a positive integer or zero |
| Calculation | Based on electron counting rules considering electronegativity | Based on the number of valence electrons involved in bonding |
| Example | Fe in Fe3+ has oxidation state +3 | Carbon in methane (CH4) has valence 4 |
| Significance | Important for redox reactions and electron transfer analysis | Important for understanding molecular structure and bonding |
Introduction to Oxidation State and Valence
Oxidation state represents the hypothetical charge an atom would have if all bonds to atoms of different elements were fully ionic, serving as a useful tool for electron accounting in redox reactions. Valence refers to the number of electrons an atom can share, gain, or lose to form chemical bonds, reflecting its bonding capacity and chemical reactivity. Understanding the distinction between oxidation state and valence aids in predicting molecular structure, reaction mechanisms, and compound stability.
Defining Oxidation State
Oxidation state is a formal charge assigned to an atom in a molecule, reflecting the number of electrons lost or gained compared to its elemental form, and is crucial for understanding redox reactions. Unlike valence, which describes the number of bonds an atom can form based on its outer electrons, oxidation state can be positive, negative, or zero depending on electron transfer in compounds. Accurately determining oxidation states involves assigning electron pairs in bonds to the more electronegative atom, providing a systematic method for tracking electron flow in chemical processes.
Understanding Valence in Chemistry
Valence in chemistry refers to the number of electrons an atom can gain, lose, or share to form chemical bonds, reflecting its bonding capacity and electron arrangement. Unlike oxidation state, which indicates the hypothetical charge an atom would have if all bonds were ionic, valence provides insight into an element's typical bonding behavior and chemical properties. Understanding valence helps predict molecule formation, reactivity, and the structure of compounds across different groups of the periodic table.
Key Differences Between Oxidation State and Valence
Oxidation state refers to the hypothetical charge an atom would have if all bonds were ionic, reflecting electron loss or gain during chemical reactions, whereas valence indicates the number of electrons involved in bonding or available for bonding by an atom. The oxidation state can be positive, negative, or zero and is assigned based on a set of rules, while valence is typically a positive integer showing an atom's combining capacity. Oxidation states vary within different compounds for the same element, but valence remains more consistent, representing the element's bonding behavior in molecules.
Determining Oxidation States: Rules and Examples
Determining oxidation states involves assigning charges to atoms based on a set of rules: the oxidation state of an element in its standard state is zero, hydrogen usually has +1, oxygen typically -2, and the sum of oxidation states in a neutral compound equals zero. For example, in H2O, hydrogen has an oxidation state of +1 and oxygen -2, resulting in a net charge of zero. Transition metals often exhibit multiple oxidation states, which are identified by the charge balance in coordination complexes or redox reactions.
Calculating Valence of Elements
Calculating the valence of elements involves determining the number of electrons an atom can gain, lose, or share to form chemical bonds, which typically corresponds to the group number in the periodic table for main-group elements. Valence is distinct from oxidation state, as valence represents bonding capacity, whereas oxidation state reflects the charge of an atom in a compound based on electron transfer. Precise calculation of valence requires analyzing electronic configuration and chemical behavior, especially for transition metals where variable valence is common.
Oxidation State in Redox Reactions
Oxidation state refers to the hypothetical charge an atom would have if all bonds to atoms of different elements were 100% ionic, crucial for tracking electron transfer in redox reactions. It quantifies the degree of oxidation or reduction of an element within a compound, helping to identify electron loss or gain during chemical changes. Precise determination of oxidation states enables understanding of reaction mechanisms and predicting product formation in redox chemistry.
Role of Valence in Chemical Bonding
Valence represents the number of electrons an atom can lose, gain, or share to form chemical bonds, directly determining the molecule's structure and stability. Its role is crucial in predicting bond formation patterns and molecular geometry, influencing reactivity and compound properties. Unlike oxidation state, which is a theoretical charge, valence reflects actual bonding capacity essential for understanding chemical interactions.
Common Misconceptions: Oxidation State vs Valence
Oxidation state represents the hypothetical charge an atom would have if all bonds were ionic, while valence refers to the number of electrons an atom uses to form bonds. A common misconception is treating oxidation state and valence as interchangeable terms, despite their distinct chemical meanings and applications. Valence typically defines bonding capacity, whereas oxidation states help track electron transfer in redox reactions.
Summary: Practical Implications in Chemistry
Oxidation state quantifies the hypothetical charge an atom would have if all bonds were ionic, providing a standardized method for tracking electron transfer in redox reactions, essential for balancing chemical equations and predicting reaction outcomes. Valence describes the number of chemical bonds an atom can form, reflecting its bonding capacity and contributing to molecular structure determination. Understanding both concepts facilitates accurate interpretation of compound reactivity, coordination chemistry, and catalysis mechanisms in practical chemical applications.
Oxidation state Infographic
 libterm.com
libterm.com