Molecular structures form the foundation of all chemical compounds, influencing their properties and interactions at the atomic level. Understanding the behavior of molecules is essential for advancements in fields such as medicine, materials science, and biochemistry. Explore this article to discover how molecular science impacts your daily life and future technological innovations.
Table of Comparison
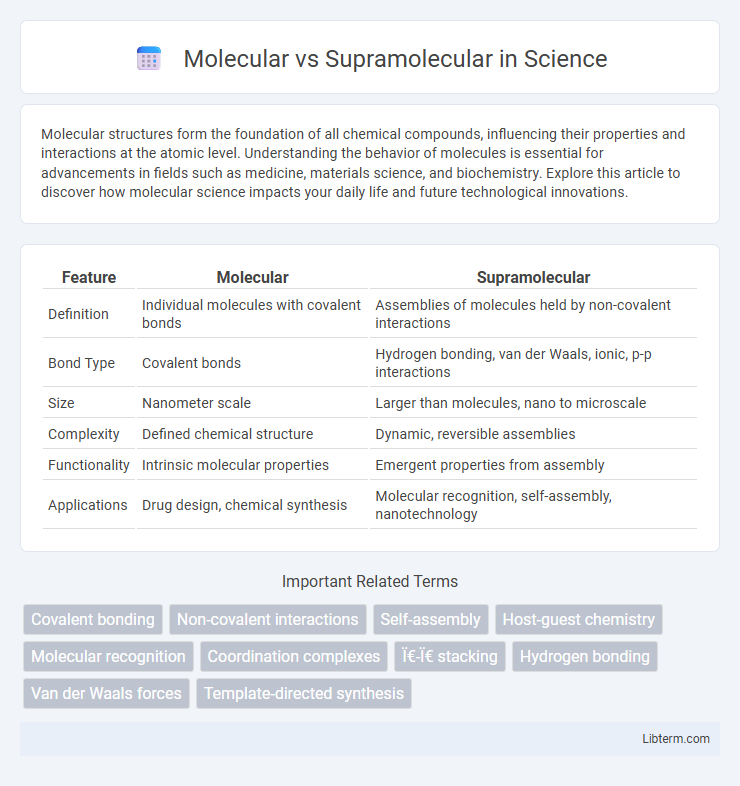
| Feature | Molecular | Supramolecular |
|---|---|---|
| Definition | Individual molecules with covalent bonds | Assemblies of molecules held by non-covalent interactions |
| Bond Type | Covalent bonds | Hydrogen bonding, van der Waals, ionic, p-p interactions |
| Size | Nanometer scale | Larger than molecules, nano to microscale |
| Complexity | Defined chemical structure | Dynamic, reversible assemblies |
| Functionality | Intrinsic molecular properties | Emergent properties from assembly |
| Applications | Drug design, chemical synthesis | Molecular recognition, self-assembly, nanotechnology |
Introduction to Molecular and Supramolecular Chemistry
Molecular chemistry studies the properties and reactions of molecules formed by covalent bonds, emphasizing the individual molecular structure and behavior. Supramolecular chemistry explores non-covalent interactions such as hydrogen bonding, van der Waals forces, and electrostatic effects that organize molecules into larger, functional assemblies. Understanding these interactions allows chemists to design complex architectures with applications in catalysis, drug delivery, and materials science.
Defining Molecular Chemistry: Key Concepts
Molecular chemistry centers on the study of molecules and their intrinsic properties, focusing on atomic composition, covalent bonding, and electronic structure that dictate molecular behavior. It involves understanding how atoms combine to form discrete units with defined molecular geometry and reactivity patterns. In contrast, supramolecular chemistry examines the non-covalent interactions between multiple molecules, emphasizing molecular recognition and assembly processes beyond individual molecular frameworks.
Understanding Supramolecular Chemistry
Supramolecular chemistry studies the organized structures formed by non-covalent interactions between molecules, unlike molecular chemistry which focuses on covalent bonds within single molecules. Understanding supramolecular chemistry involves exploring hydrogen bonding, metal coordination, and van der Waals forces that drive self-assembly and molecular recognition. This field enables the design of complex functional systems such as molecular machines, sensors, and drug delivery vehicles by manipulating intermolecular forces.
Historical Development and Milestones
The historical development of molecular chemistry began in the early 19th century with Amedeo Avogadro's hypothesis and culminated in the discovery of molecular structures through techniques like X-ray crystallography in the early 20th century. Supramolecular chemistry emerged in the late 20th century, marked by the identification of non-covalent interactions and the pioneering work of Jean-Marie Lehn, who received the Nobel Prize in Chemistry in 1987 for his research on molecular recognition and self-assembly. Key milestones include the synthesis of crown ethers, the development of host-guest chemistry, and the integration of supramolecular principles into nanotechnology and materials science.
Fundamental Differences: Molecular vs Supramolecular
Molecular systems consist of individual molecules held together by covalent bonds, defining their intrinsic chemical identity and properties at the atomic level. Supramolecular systems arise from the organized assembly of multiple molecules through non-covalent interactions such as hydrogen bonding, van der Waals forces, and electrostatic interactions, enabling dynamic and reversible structural complexity. This fundamental distinction highlights the hierarchical nature of supramolecular chemistry, emphasizing collective behavior beyond single-molecule characteristics.
Bonding and Interactions: Covalent vs Non-Covalent
Molecular systems are characterized by covalent bonds, where atoms share electron pairs, resulting in strong, stable connections essential for defining molecular structure and properties. Supramolecular systems rely predominantly on non-covalent interactions such as hydrogen bonding, van der Waals forces, and electrostatic interactions, which are weaker and reversible, allowing dynamic assembly and disassembly. These distinct bonding modalities govern the stability, functionality, and responsiveness of molecular versus supramolecular architectures in chemical and biological contexts.
Applications in Materials Science and Nanotechnology
Molecular systems consist of individual molecules with fixed covalent bonds, enabling precise chemical synthesis for organic electronics and drug delivery platforms. Supramolecular assemblies rely on non-covalent interactions like hydrogen bonding and p-p stacking, facilitating dynamic, self-healing materials and stimuli-responsive nanodevices. Applications in nanotechnology leverage supramolecular chemistry to create adaptable nanoscale architectures, while molecular approaches provide stability and specificity in sensor development and nanofabrication.
Role in Biological Systems and Medicine
Molecular structures form the fundamental basis of biomolecules like proteins and nucleic acids, essential for cellular function and drug design. Supramolecular assemblies enable complex biological processes such as DNA replication, signal transduction, and enzyme catalysis through non-covalent interactions. In medicine, supramolecular chemistry advances targeted drug delivery, diagnostics, and biomimetic materials by harnessing reversible and dynamic molecular recognition.
Challenges and Future Perspectives
Molecular systems face challenges in precise control over individual molecule behavior, stability, and scalability, limiting their application in complex functions. Supramolecular chemistry addresses these issues by harnessing non-covalent interactions for dynamic, reversible assemblies, yet challenges remain in achieving predictable self-assembly and robustness under varying conditions. Future perspectives emphasize integrating molecular precision with supramolecular complexity to develop responsive materials and advanced nanoscale devices.
Conclusion: Harmonizing Molecular and Supramolecular Approaches
Harmonizing molecular and supramolecular approaches enhances the design of complex systems by integrating precise molecular recognition with dynamic self-assembly processes. This synergy enables the development of advanced materials and functional architectures with tailored properties and responsive behaviors. Future innovations rely on balancing covalent bonding precision with non-covalent interaction versatility to achieve unprecedented control in chemical and biological applications.
Molecular Infographic
 libterm.com
libterm.com