Polar regions experience extreme temperatures and unique wildlife adaptations essential for maintaining global ecological balance. Understanding the impact of climate change on these fragile environments reveals critical insights into rising sea levels and shifting weather patterns. Explore the rest of this article to learn how your actions can influence the future of polar ecosystems.
Table of Comparison
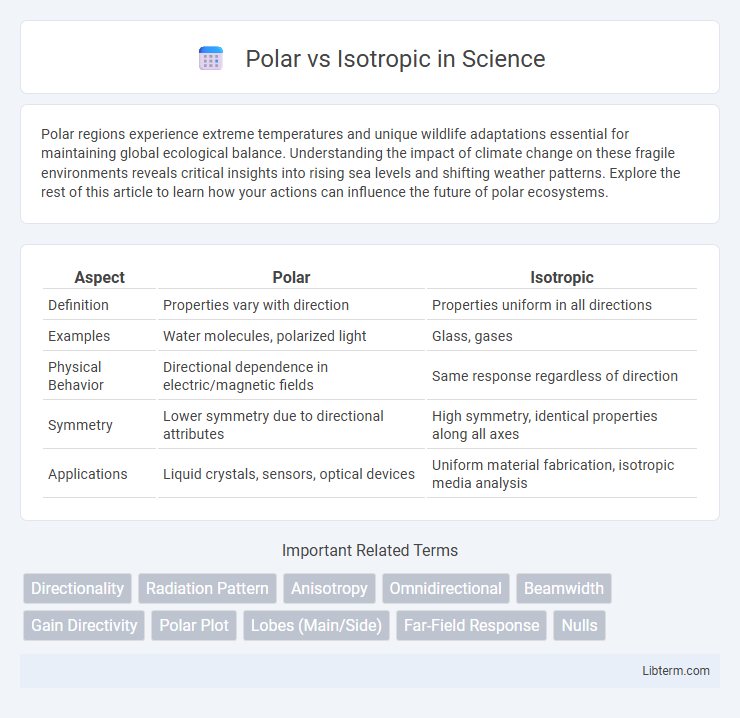
| Aspect | Polar | Isotropic |
|---|---|---|
| Definition | Properties vary with direction | Properties uniform in all directions |
| Examples | Water molecules, polarized light | Glass, gases |
| Physical Behavior | Directional dependence in electric/magnetic fields | Same response regardless of direction |
| Symmetry | Lower symmetry due to directional attributes | High symmetry, identical properties along all axes |
| Applications | Liquid crystals, sensors, optical devices | Uniform material fabrication, isotropic media analysis |
Introduction: Understanding Polar and Isotropic Concepts
Polar materials exhibit directional dependence in their physical properties, meaning their characteristics vary based on orientation within the material. In contrast, isotropic materials display uniform properties regardless of direction, resulting in consistent behavior under varying conditions. Understanding the distinction between polar and isotropic is crucial in fields like material science and optics, where material anisotropy influences performance and application outcomes.
Key Definitions: Polar vs. Isotropic Explained
Polar materials exhibit directional dependence in their physical properties, meaning characteristics such as electrical conductivity or refractive index vary based on the orientation within the material. Isotropic materials have uniform properties in all directions, ensuring consistent behavior regardless of measurement angle or axis. Understanding the distinction between polar and isotropic materials is crucial for applications in optics, electronics, and materials science where anisotropy impacts performance and design.
Historical Background and Scientific Relevance
Polar and isotropic materials have distinct historical backgrounds rooted in early 20th-century physics and chemistry research. Polar substances, characterized by permanent dipole moments, were first thoroughly studied through molecular spectroscopy and electrostatics, while isotropic materials, exhibiting uniform properties in all directions, have long been foundational in classical mechanics and materials science. Their scientific relevance persists in fields such as crystallography, liquid crystal technology, and electromagnetic theory, where understanding anisotropy versus isotropy informs material design and application.
Physical Properties Comparison
Polar molecules exhibit distinct dipole moments due to uneven charge distribution, resulting in higher boiling points and greater solubility in polar solvents compared to isotropic molecules, which have uniform physical properties in all directions due to symmetrical charge distribution. The anisotropic nature of polar compounds influences their viscosity and dielectric constants, whereas isotropic substances typically display uniform viscosity and dielectric behavior regardless of orientation. These fundamental differences in molecular polarity directly impact thermal conductivity and refractive indices, making polar substances more responsive to electric fields than isotropic materials.
Polar and Isotropic in Materials Science
Polar materials exhibit a permanent electric dipole moment due to asymmetric charge distribution, influencing properties like dielectric behavior and piezoelectricity. Isotropic materials possess uniform properties in all directions, resulting from their symmetrical atomic or molecular structure, which affects mechanical, thermal, and optical behavior. Understanding the distinction between polar and isotropic materials is crucial for applications in sensors, actuators, and electronic devices where directional properties impact performance.
Applications in Engineering and Technology
Polar materials exhibit directional dependence in properties like electrical conductivity and permittivity, making them essential in applications such as piezoelectric sensors, liquid crystal displays, and anisotropic conductive films in electronics. Isotropic materials maintain uniform properties regardless of direction, which is crucial in structural engineering components, aerospace materials, and pressure vessel design for predictable mechanical behavior. Engineering applications leverage the unique directional characteristics of polar materials for advanced functionality while relying on isotropic materials for consistent performance and reliability.
Advantages and Disadvantages of Polar vs Isotropic
Polar materials exhibit directional dependence in their properties, offering advantages such as enhanced sensitivity and performance in sensors and actuators, but they may suffer from anisotropic mechanical weaknesses and fabrication complexity. Isotropic materials provide uniform properties in all directions, simplifying design and manufacturing processes, yet they often lack the tailored performance benefits that polar materials deliver in specialized applications. Choosing between polar and isotropic materials hinges on balancing the need for directional functionality against ease of production and material robustness.
Common Examples and Case Studies
Polar materials, such as water and hydrogen fluoride, exhibit permanent dipole moments resulting in strong intermolecular forces, which are evident in their high boiling points and solubility in polar solvents. Isotropic substances like noble gases and nonpolar hydrocarbons show uniform properties in all directions due to the absence of permanent dipole moments, making them ideal for applications requiring consistent physical properties, such as in optical fibers or lubricants. Case studies of polar solvents in chemical reactions demonstrate enhanced reaction rates through dipole-dipole interactions, while isotropic materials are preferred in electronic insulation where directional uniformity is critical.
Challenges and Considerations in Real-World Uses
Polar materials exhibit directional properties that complicate their integration into devices requiring uniform responses, posing challenges in fabrication and performance consistency. Isotropic materials, while offering uniformity, may lack the specialized functionalities needed in advanced applications, leading to trade-offs in efficiency and effectiveness. Key considerations include managing anisotropic stress distribution in polar substances and ensuring isotropic materials meet functional demands without sacrificing structural integrity.
Conclusion: Choosing Between Polar and Isotropic
Choosing between polar and isotropic configurations depends on the specific application requirements and material behavior. Polar materials exhibit direction-dependent properties ideal for sensors and anisotropic conductive films, while isotropic materials provide uniform characteristics suitable for structural components and flexible substrates. Understanding the performance demands and environmental conditions ensures optimal selection between polar and isotropic options.
Polar Infographic
 libterm.com
libterm.com