Radioactive decay is the spontaneous process by which unstable atomic nuclei lose energy by emitting radiation, transforming into more stable elements over time. This phenomenon plays a crucial role in fields such as nuclear medicine, archaeology, and energy production. Discover how radioactive decay impacts your life and the world around you by exploring the rest of this article.
Table of Comparison
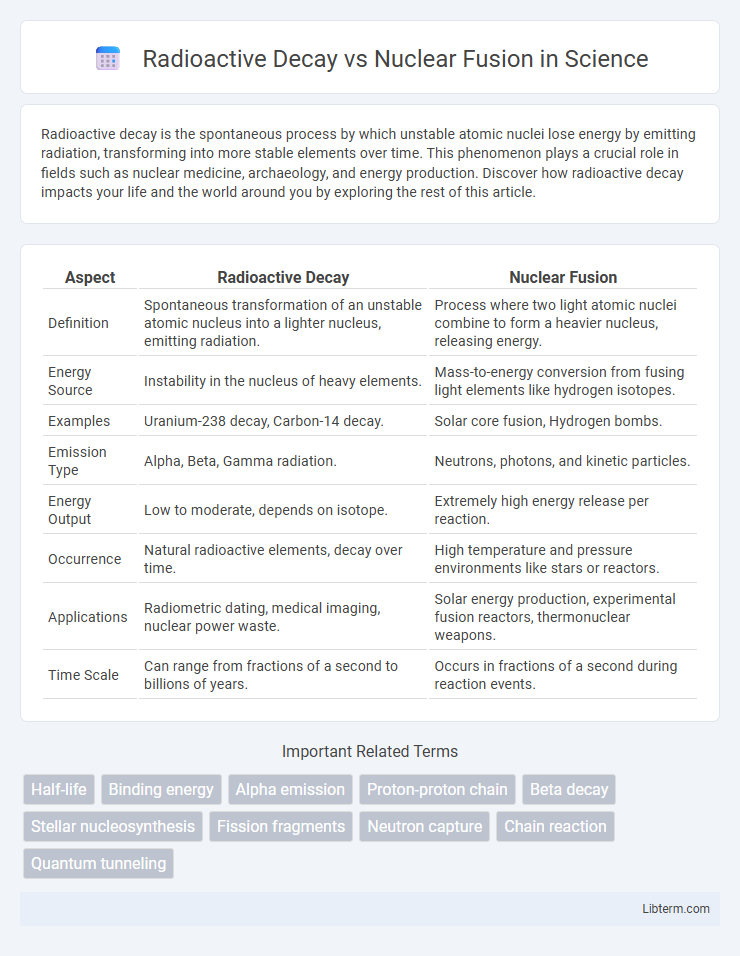
| Aspect | Radioactive Decay | Nuclear Fusion |
|---|---|---|
| Definition | Spontaneous transformation of an unstable atomic nucleus into a lighter nucleus, emitting radiation. | Process where two light atomic nuclei combine to form a heavier nucleus, releasing energy. |
| Energy Source | Instability in the nucleus of heavy elements. | Mass-to-energy conversion from fusing light elements like hydrogen isotopes. |
| Examples | Uranium-238 decay, Carbon-14 decay. | Solar core fusion, Hydrogen bombs. |
| Emission Type | Alpha, Beta, Gamma radiation. | Neutrons, photons, and kinetic particles. |
| Energy Output | Low to moderate, depends on isotope. | Extremely high energy release per reaction. |
| Occurrence | Natural radioactive elements, decay over time. | High temperature and pressure environments like stars or reactors. |
| Applications | Radiometric dating, medical imaging, nuclear power waste. | Solar energy production, experimental fusion reactors, thermonuclear weapons. |
| Time Scale | Can range from fractions of a second to billions of years. | Occurs in fractions of a second during reaction events. |
Introduction to Radioactive Decay and Nuclear Fusion
Radioactive decay is the spontaneous transformation of an unstable atomic nucleus into a more stable one, emitting ionizing radiation such as alpha, beta, or gamma particles. Nuclear fusion involves the process where two light atomic nuclei combine to form a heavier nucleus, releasing immense energy due to mass-to-energy conversion as described by Einstein's equation E=mc2. Both processes are fundamental to nuclear physics, with radioactive decay governing natural element transmutation and nuclear fusion powering stars and potential clean energy sources.
Fundamental Principles of Radioactive Decay
Radioactive decay is governed by the fundamental principle of the spontaneous transformation of an unstable atomic nucleus into a more stable configuration, emitting ionizing radiation in the form of alpha particles, beta particles, or gamma rays. This process follows an exponential decay law characterized by a half-life, which quantifies the time taken for half of the radioactive nuclei in a sample to decay, independent of external conditions. Unlike nuclear fusion, which involves joining nuclei to release energy, radioactive decay is a unidirectional, stochastic event intrinsic to the individual properties of each radionuclide.
Core Mechanisms Behind Nuclear Fusion
Nuclear fusion involves the core mechanism of fusing light atomic nuclei, such as hydrogen isotopes, into heavier nuclei, releasing vast amounts of energy due to mass-to-energy conversion described by Einstein's equation E=mc2. This process occurs under extreme temperatures and pressures, enabling the overcoming of electrostatic repulsion between positively charged nuclei through quantum tunneling effects. In contrast, radioactive decay is a spontaneous process where unstable nuclei emit radiation to reach a more stable state without the need for external conditions like those essential in nuclear fusion.
Energy Production: Decay vs. Fusion
Radioactive decay releases energy through the spontaneous transformation of unstable atomic nuclei, producing relatively low but continuous power over long periods, mainly utilized in nuclear batteries and radiometric dating. Nuclear fusion generates immense energy by combining light atomic nuclei, such as hydrogen isotopes, resulting in a high-energy yield with potential for sustainable, clean power in future fusion reactors. The energy density of fusion surpasses that of radioactive decay by several orders of magnitude, making fusion a promising solution for large-scale energy production.
Types of Radioactive Decay Processes
Radioactive decay involves spontaneous nuclear transformations such as alpha decay, beta decay (including beta-minus and beta-plus), and gamma decay, each characterized by the emission of specific particles or radiation that change the atomic nucleus. Alpha decay releases helium nuclei, beta decay emits electrons or positrons altering the neutron-to-proton ratio, and gamma decay involves high-energy photon emission without altering the nucleus's composition. Unlike nuclear fusion, which combines light nuclei under extreme temperature and pressure to form heavier elements and releases significant energy, radioactive decay processes are governed by nuclear instability and result in the transmutation of elements over time.
Key Stages in Nuclear Fusion Reactions
Nuclear fusion reactions primarily involve the stages of overcoming the Coulomb barrier, where atomic nuclei approach closely enough for the strong nuclear force to bind them together, releasing immense energy. This process begins with the high-temperature plasma state, enabling nuclei like hydrogen isotopes to collide and fuse into heavier elements such as helium. Unlike radioactive decay, which is spontaneous and involves unstable nuclei emitting particles, fusion is a carefully controlled reaction requiring extreme conditions, often replicated in tokamaks or inertial confinement fusion devices.
Occurrence in Nature and Practical Applications
Radioactive decay naturally occurs in unstable isotopes such as uranium-238 and carbon-14, releasing alpha, beta, or gamma radiation with predictable half-lives essential in radiometric dating and medical diagnostics. Nuclear fusion primarily powers stars like the sun, where hydrogen nuclei fuse under extreme heat and pressure to form helium, generating vast energy but remains challenging for controlled terrestrial applications. Practical applications of fusion focus on experimental reactors like ITER aiming for clean energy, while radioactive decay is harnessed in smoke detectors, cancer treatment, and geological dating.
Safety Concerns and Environmental Impacts
Radioactive decay involves the spontaneous emission of particles from unstable nuclei, posing risks such as radiation exposure and long-term environmental contamination. Nuclear fusion produces energy by combining light nuclei, resulting in minimal radioactive waste and a significantly lower risk of nuclear accidents. Fusion's byproducts primarily include harmless helium, making it a safer and more environmentally friendly energy source compared to radioactive decay-based processes.
Technological Challenges and Advancements
Radioactive decay involves the spontaneous emission of particles from unstable isotopes, while nuclear fusion requires the merging of light nuclei under extreme temperatures and pressure to release energy. Technological challenges in fusion include maintaining plasma stability and achieving net energy gain, with advancements such as magnetic confinement in tokamaks and inertial confinement fusion lasers significantly progressing research. In contrast, handling radioactive decay centers around controlling radiation safely and improving detection technologies for medical, industrial, and energy applications.
Future Prospects in Energy and Scientific Research
Radioactive decay offers reliable baseline data essential for nuclear medicine and radiometric dating, while nuclear fusion holds transformative potential for clean, virtually limitless energy with minimal radioactive waste. Advances in fusion technology, such as tokamaks and inertial confinement, aim to achieve sustained reactions that could revolutionize power generation by the mid-21st century. Ongoing research in both fields contributes to deeper understanding of atomic processes, with fusion research expected to drive innovation in materials science, plasma physics, and sustainable energy solutions.
Radioactive Decay Infographic
 libterm.com
libterm.com