Enantiomers are pairs of molecules that are mirror images of each other but cannot be superimposed, playing a crucial role in chemistry and pharmaceuticals due to their different biological activities. Understanding the distinct properties of enantiomers helps optimize drug efficacy and safety by targeting specific interactions within the body. Explore the rest of this article to discover how enantiomers influence chemical reactions and medical treatments.
Table of Comparison
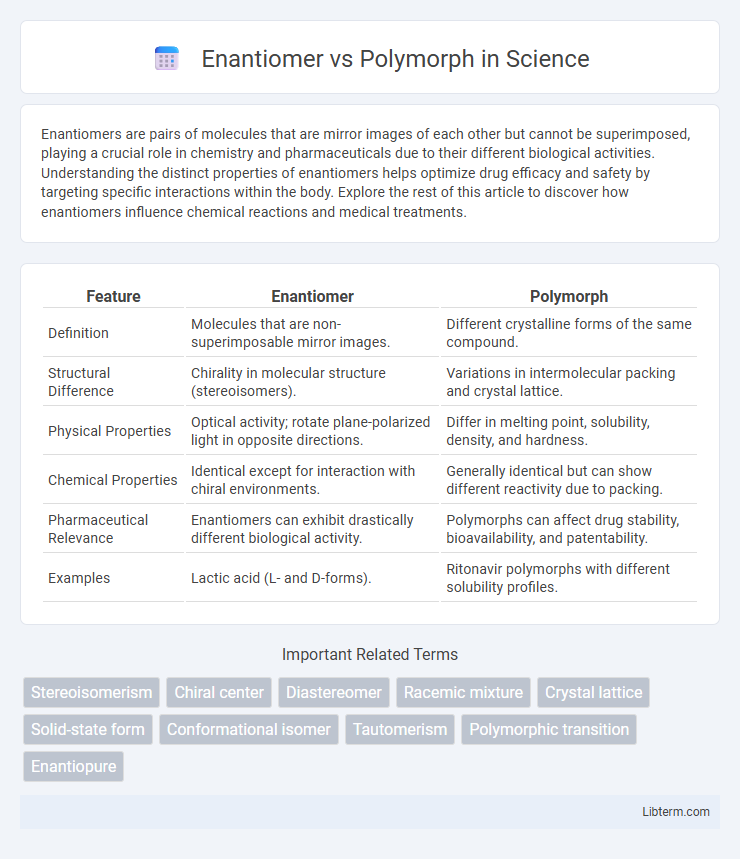
| Feature | Enantiomer | Polymorph |
|---|---|---|
| Definition | Molecules that are non-superimposable mirror images. | Different crystalline forms of the same compound. |
| Structural Difference | Chirality in molecular structure (stereoisomers). | Variations in intermolecular packing and crystal lattice. |
| Physical Properties | Optical activity; rotate plane-polarized light in opposite directions. | Differ in melting point, solubility, density, and hardness. |
| Chemical Properties | Identical except for interaction with chiral environments. | Generally identical but can show different reactivity due to packing. |
| Pharmaceutical Relevance | Enantiomers can exhibit drastically different biological activity. | Polymorphs can affect drug stability, bioavailability, and patentability. |
| Examples | Lactic acid (L- and D-forms). | Ritonavir polymorphs with different solubility profiles. |
Introduction to Enantiomers and Polymorphs
Enantiomers are pairs of molecules that are non-superimposable mirror images of each other, differing primarily in the spatial arrangement of atoms around a chiral center, which significantly impacts their biological activity and pharmacokinetics. Polymorphs refer to different crystalline forms of the same compound, exhibiting distinct physical properties such as melting point, solubility, and stability due to variations in molecular packing. Understanding the distinction between enantiomers and polymorphs is critical in pharmaceutical development to optimize drug efficacy and manufacturing processes.
Defining Enantiomers: Key Characteristics
Enantiomers are chiral molecules that exist as non-superimposable mirror images, exhibiting identical physical properties except for their interaction with plane-polarized light and chiral environments. Each enantiomer rotates plane-polarized light in opposite directions, a property known as optical activity, which is crucial in pharmaceuticals for determining drug efficacy and safety. Unlike polymorphs, which differ in crystal structure, enantiomers are indistinguishable in chemical composition but differ fundamentally in spatial arrangement, impacting biological activity significantly.
Understanding Polymorphs: Core Features
Polymorphs refer to different crystal structures of the same chemical compound, which significantly impact physical properties such as melting point, solubility, and stability. Unlike enantiomers, which are non-superimposable mirror images with identical physical properties except for optical activity, polymorphs exhibit distinct packing arrangements affecting drug formulation and bioavailability. Understanding polymorphs is crucial in pharmaceuticals to optimize therapeutic efficacy and ensure patent protection.
Chemical Structure Differences: Enantiomer vs Polymorph
Enantiomers are stereoisomers that are non-superimposable mirror images of each other, differing specifically in the spatial arrangement of atoms around a chiral center without altering the molecular formula or connectivity. Polymorphs, on the other hand, are different crystal forms of the same compound, where molecules share an identical chemical structure but vary in their packing arrangement and intermolecular interactions. The key chemical structure difference lies in enantiomers having distinct three-dimensional configurations due to chirality, whereas polymorphs maintain the same molecular geometry but differ in solid-state organization.
Formation Mechanisms: How Enantiomers and Polymorphs Arise
Enantiomers form through chiral synthesis or resolution processes that produce non-superimposable mirror-image molecules, often influenced by asymmetric catalysts or chiral environments during chemical reactions. Polymorphs arise from different molecular packing arrangements or conformations in the solid state, driven by variations in crystallization conditions such as temperature, pressure, and solvent choice. Both phenomena highlight the critical role of stereochemistry and thermodynamics in determining molecular structure and physical properties.
Pharmaceutical Importance of Each
Enantiomers in pharmaceuticals are crucial due to their distinct biological activities despite identical molecular formulas, impacting drug efficacy and safety by interacting differently with chiral biological targets. Polymorphs affect drug solubility, stability, and bioavailability, influencing formulation development and manufacturing consistency for optimal therapeutic outcomes. Understanding both enantiomeric purity and polymorphic forms ensures precise dosage, enhanced drug performance, and regulatory compliance in pharmaceutical production.
Analytical Techniques for Distinguishing Enantiomers and Polymorphs
Analytical techniques for distinguishing enantiomers include chiral chromatography, circular dichroism (CD) spectroscopy, and nuclear magnetic resonance (NMR) with chiral shift reagents, which specifically target stereochemical differences. Polymorph identification relies on powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), and infrared (IR) spectroscopy to detect variations in crystal lattice and thermal properties. Advanced methods such as Raman spectroscopy and solid-state NMR provide complementary data for comprehensive characterization of both enantiomers and polymorphs in pharmaceutical and chemical research.
Impact on Drug Efficacy and Safety
Enantiomers and polymorphs significantly influence drug efficacy and safety by altering pharmacokinetics and pharmacodynamics. Enantiomers, being mirror-image molecules, can exhibit distinct therapeutic effects and toxicity profiles, with one isomer often demonstrating superior receptor binding or reduced adverse effects. Polymorphs, different crystalline forms of the same drug, impact solubility and bioavailability, thereby affecting drug absorption rates and stability, which are critical for consistent therapeutic outcomes and minimizing side effects.
Regulatory Considerations and Quality Control
Regulatory considerations for enantiomers require thorough chiral purity assessment to ensure safety and efficacy, as each enantiomer can exhibit distinct pharmacological effects. Polymorphs demand rigorous characterization and control during drug development and manufacturing, as different crystal forms can impact bioavailability, stability, and solubility. Quality control protocols for both enantiomers and polymorphs include advanced analytical techniques such as chiral chromatography and X-ray diffraction to maintain consistent drug performance and comply with regulatory standards.
Summary: Choosing the Right Approach in Drug Development
Enantiomers and polymorphs both significantly impact drug efficacy and safety, requiring careful consideration during drug development. Enantiomers refer to chiral molecules with identical molecular formulas but different spatial arrangements, often exhibiting distinct pharmacological effects, whereas polymorphs pertain to different crystalline forms of the same compound, affecting solubility and stability. Selecting the appropriate approach depends on the target therapeutic profile and regulatory requirements, ensuring optimized bioavailability and minimized adverse effects.
Enantiomer Infographic
 libterm.com
libterm.com