Ion therapy harnesses charged particles to target cancer cells with remarkable precision, minimizing damage to surrounding healthy tissue. This cutting-edge treatment improves outcomes and reduces side effects compared to traditional radiation methods. Discover how ion therapy could be a game-changer for your cancer care by exploring the rest of this article.
Table of Comparison
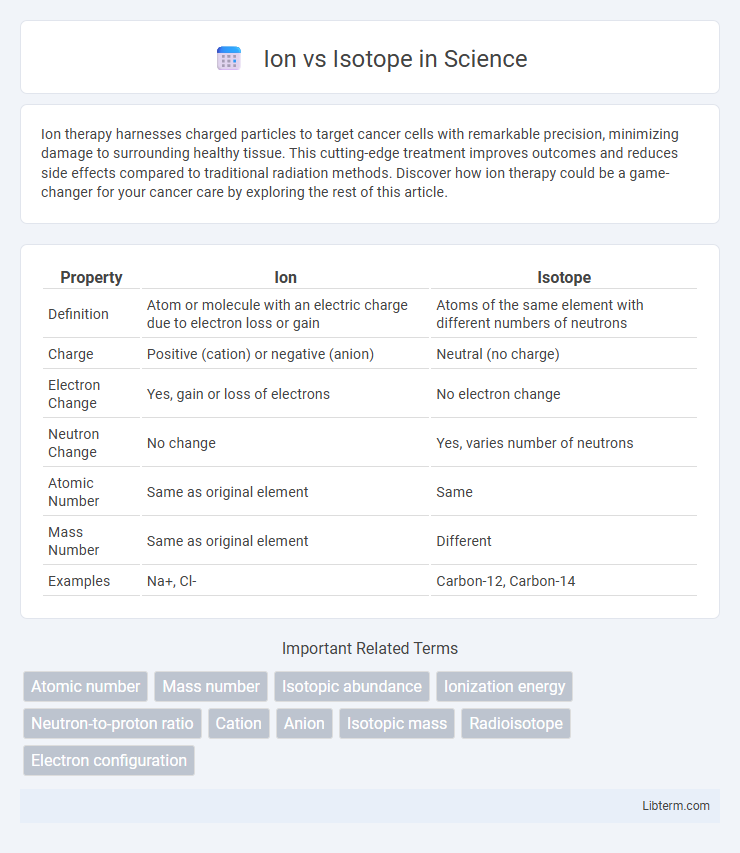
| Property | Ion | Isotope |
|---|---|---|
| Definition | Atom or molecule with an electric charge due to electron loss or gain | Atoms of the same element with different numbers of neutrons |
| Charge | Positive (cation) or negative (anion) | Neutral (no charge) |
| Electron Change | Yes, gain or loss of electrons | No electron change |
| Neutron Change | No change | Yes, varies number of neutrons |
| Atomic Number | Same as original element | Same |
| Mass Number | Same as original element | Different |
| Examples | Na+, Cl- | Carbon-12, Carbon-14 |
Understanding the Basics: What Are Ions and Isotopes?
Ions are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge, either positive (cations) or negative (anions). Isotopes are variants of a particular chemical element that share the same number of protons but differ in neutron count, affecting atomic mass without altering chemical properties. Understanding the distinction between electrical charge in ions and neutron variation in isotopes is fundamental to grasping atomic structure and behavior in chemistry.
Definition and Formation of Ions
Ions are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge, either positive (cations) or negative (anions). Isotopes, by contrast, are variants of the same element that have identical numbers of protons but different numbers of neutrons, thus differing in atomic mass but not electrical charge. Ion formation occurs through the transfer or sharing of electrons during chemical reactions or physical processes like ionization, altering the electron count without changing the nucleus.
Definition and Types of Isotopes
An ion is an atom or molecule with a net electric charge due to the loss or gain of electrons, classified as cations (positive) or anions (negative). Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons, affecting their atomic mass but not their chemical properties. Types of isotopes include stable isotopes, which do not decay over time, and radioactive isotopes, which undergo radioactive decay and are used in medical imaging and radiometric dating.
Key Differences: Ion vs Isotope
Ions are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge, while isotopes are variants of the same element with different numbers of neutrons, leading to variations in atomic mass but no charge difference. The key difference lies in charge alteration for ions versus neutron number variation for isotopes. Ions affect chemical reactivity due to their charge, whereas isotopes primarily influence atomic mass and nuclear properties without changing chemical behavior.
Atomic Structure: Electrons, Protons, and Neutrons
Ions and isotopes differ fundamentally in atomic structure: ions have an unequal number of electrons compared to protons, creating a net electric charge, while isotopes contain the same number of protons but different numbers of neutrons, affecting atomic mass without altering charge. Electrons in ions are gained or lost, influencing chemical reactivity and bonding, whereas isotopes vary neutron count, impacting nuclear stability and radioactive properties. Protons remain constant in both ions and isotopes, defining the element's identity in the periodic table.
Real-World Examples of Ions
Sodium ions (Na+) play a crucial role in nerve signal transmission and muscle contraction, highlighting their significance in biological systems. Chloride ions (Cl-) maintain fluid balance in cells and are essential in digestive processes through hydrochloric acid in the stomach. Unlike isotopes, which vary in neutron number, ions differ by electron count, directly impacting their chemical reactivity in real-world applications such as battery technology and water purification.
Real-World Applications of Isotopes
Isotopes play a crucial role in real-world applications such as medical diagnostics, where radioactive isotopes like iodine-131 are used in thyroid imaging and treatment, and carbon-14 is employed in radiocarbon dating to determine the age of archaeological samples. Unlike ions, which involve electron loss or gain affecting chemical reactivity, isotopes differ in neutron number, impacting nuclear properties without changing chemical behavior. Stable isotopes are also vital in environmental studies and tracing biochemical pathways, demonstrating their broad utility across scientific fields.
Chemical Properties: Impact of Ions and Isotopes
Ions exhibit altered chemical properties due to the gain or loss of electrons, which directly affects their reactivity and bonding behavior in chemical reactions. Isotopes maintain nearly identical chemical properties since they share the same number of protons and electrons, but differences in neutron count influence nuclear stability and mass-dependent physical properties. The impact of ions is evident in changes to electrical conductivity and solubility, while isotopes are primarily involved in tracing reaction mechanisms and dating materials through isotope labeling.
Importance in Science and Industry
Ions play a critical role in electrochemistry and battery technology, enabling energy storage and transfer through charged particle movement, which is essential for portable electronics and electric vehicles. Isotopes serve as vital tools in medical diagnostics and treatment, such as in radioactive tracers and cancer radiotherapy, providing targeted and efficient healthcare solutions. Both ions and isotopes are fundamental in environmental science for tracing chemical pathways and dating materials, advancing research and industrial applications across multiple fields.
Summary: Ion vs Isotope Comparison Chart
Ions are atoms or molecules that have gained or lost electrons, resulting in a net electric charge, while isotopes are variants of a particular chemical element with the same number of protons but different numbers of neutrons. The Ion vs Isotope Comparison Chart highlights that ions differ in electron count affecting charge, whereas isotopes differ in neutron count affecting atomic mass. Key distinctions include ions participating in chemical reactions due to charge differences, while isotopes exhibit variations mainly in nuclear properties and stability.
Ion Infographic
 libterm.com
libterm.com