Metastable states occur when a system remains in a non-equilibrium condition for an extended period before transitioning to a more stable state. Understanding these states is crucial in fields like physics, chemistry, and materials science, as they influence reaction dynamics and material properties. Explore the rest of the article to discover how metastability impacts your applications and everyday phenomena.
Table of Comparison
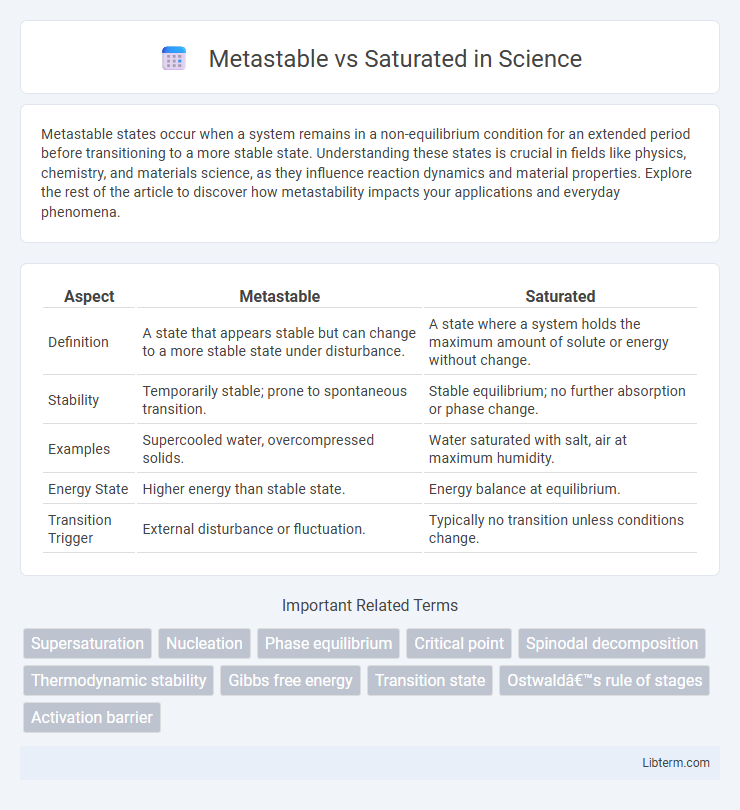
| Aspect | Metastable | Saturated |
|---|---|---|
| Definition | A state that appears stable but can change to a more stable state under disturbance. | A state where a system holds the maximum amount of solute or energy without change. |
| Stability | Temporarily stable; prone to spontaneous transition. | Stable equilibrium; no further absorption or phase change. |
| Examples | Supercooled water, overcompressed solids. | Water saturated with salt, air at maximum humidity. |
| Energy State | Higher energy than stable state. | Energy balance at equilibrium. |
| Transition Trigger | External disturbance or fluctuation. | Typically no transition unless conditions change. |
Introduction to Metastable and Saturated States
Metastable states refer to non-equilibrium conditions where a system remains temporarily stable despite being in a higher energy state than the saturated state, which represents true thermodynamic equilibrium. In thermodynamics, a saturated state is characterized by a phase change boundary, such as the coexistence of liquid and vapor at a specific pressure and temperature, whereas metastable states exist beyond these boundaries, like superheated liquids or supercooled vapors. Understanding the distinctions between metastable and saturated states is crucial for applications in materials science, chemical engineering, and phase transition analysis.
Defining Metastable States
Metastable states refer to non-equilibrium conditions where a system remains stable for extended periods despite not being in its lowest energy configuration, contrasting with saturated states that represent maximum solute concentration at equilibrium. Defining metastable states involves understanding their kinetic stability, where energy barriers prevent immediate transition to the stable phase, such as supercooled liquids or supersaturated solutions. These states are crucial in materials science and chemistry because they influence nucleation rates and phase transformations under controlled conditions.
Characteristics of Saturated States
Saturated states are characterized by equilibrium conditions where liquid and vapor phases coexist at a specific temperature and pressure, resulting in maximum energy storage capacity without a phase change. These states exhibit constant temperature and pressure during phase transitions, making them critical in thermodynamic processes such as boiling and condensation. Saturated states define the boundary between subcooled liquid and superheated vapor, optimizing energy transfer efficiency in heating and cooling systems.
Key Differences Between Metastable and Saturated
Metastable refers to a state of a system that is stable under small disturbances but may transition to a more stable state when triggered, whereas saturated describes a condition where a substance or system is fully occupied or filled, such as a solution holding the maximum solute at a given temperature. Metastable states are often temporary and exist under non-equilibrium conditions, while saturated states represent equilibrium where no further change occurs unless conditions are altered. The key difference lies in metastability's potential for change under perturbation versus saturation's limit state defined by equilibrium constraints.
Physical Examples of Metastable Systems
Metastable systems maintain a non-equilibrium state that appears stable over time until a disturbance triggers transition to a more stable phase, such as supercooled water remaining liquid below freezing point or diamond persisting despite graphite being thermodynamically more stable at standard conditions. In contrast, saturated systems represent equilibrium states where phase changes occur without altering temperature or pressure, like water in a saturated vapor-liquid equilibrium. These physical examples highlight the distinction between metastable conditions, which are kinetically hindered, and saturated states defined by thermodynamic balance.
Real-world Applications of Saturated States
Saturated states are critical in real-world applications such as refrigeration, where refrigerants operate at saturated vapor or liquid conditions to maximize heat transfer efficiency in heat exchangers. In steam power plants, saturated steam is used to optimize turbine performance by controlling moisture content and energy extraction. Chemical processes also rely on saturated solutions to maintain precise reaction conditions and ensure product consistency.
Energy Dynamics in Metastable vs Saturated Systems
Metastable systems possess higher internal energy and exist in a non-equilibrium state, allowing them to transition to a more stable configuration with energy release. Saturated systems, by contrast, have reached thermodynamic equilibrium where energy inputs and outputs balance, preventing spontaneous change. The energy dynamics in metastable states involve potential energy barriers and activation energy, while saturated states minimize free energy, maintaining system stability.
Stability and Transition Mechanisms
Metastable states possess higher energy but remain stable due to kinetic barriers preventing immediate transformation, while saturated states achieve maximum solute concentration, maintaining equilibrium stability. Transition mechanisms from metastable to stable states involve overcoming activation energy via nucleation and growth processes, contrasting with saturated solutions where phase changes occur upon solute or temperature variations. Understanding stability dynamics and transition kinetics aids in controlling crystallization, precipitation, and phase behavior in various chemical and physical systems.
Importance in Materials Science and Chemistry
Metastable states play a critical role in materials science and chemistry by enabling the study of non-equilibrium phases that exhibit unique mechanical, thermal, and chemical properties not found in saturated systems. Understanding the distinction between metastable and saturated conditions allows researchers to manipulate phase transitions, optimize material performance, and design advanced compounds with tailored functionalities. Precise control over metastable states facilitates innovations in catalysis, alloy development, and crystal growth, driving advancements in both fundamental research and industrial applications.
Summary: Choosing Metastable or Saturated Conditions
Choosing between metastable and saturated conditions depends on application-specific stability and thermodynamic requirements. Metastable states provide kinetic stability with the presence of supersaturation, beneficial in processes like crystallization and nucleation control. Saturated conditions represent equilibrium states with maximum solute concentration, ensuring thermodynamic stability and preventing spontaneous phase changes.
Metastable Infographic
 libterm.com
libterm.com