Pyroptotic cell death is a highly inflammatory form of programmed cell death triggered by infection or cellular stress, resulting in the release of pro-inflammatory cytokines like IL-1b and IL-18. This process plays a crucial role in eliminating infected cells and activating immune responses but can also contribute to inflammatory diseases when dysregulated. Discover how understanding pyroptotic mechanisms can impact your approaches to treating inflammation by reading the rest of this article.
Table of Comparison
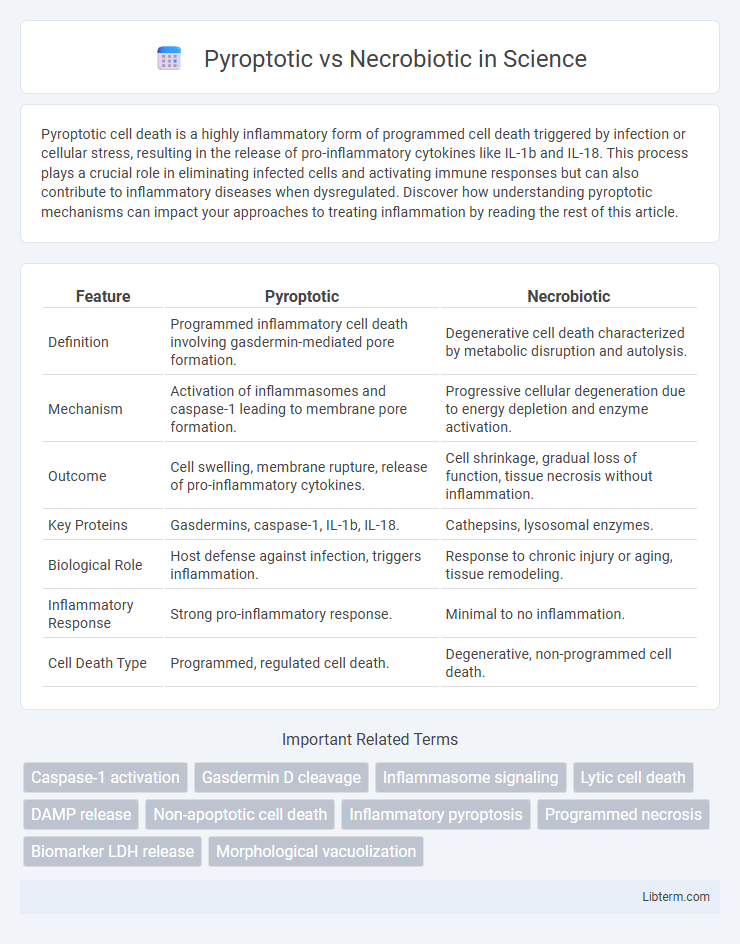
| Feature | Pyroptotic | Necrobiotic |
|---|---|---|
| Definition | Programmed inflammatory cell death involving gasdermin-mediated pore formation. | Degenerative cell death characterized by metabolic disruption and autolysis. |
| Mechanism | Activation of inflammasomes and caspase-1 leading to membrane pore formation. | Progressive cellular degeneration due to energy depletion and enzyme activation. |
| Outcome | Cell swelling, membrane rupture, release of pro-inflammatory cytokines. | Cell shrinkage, gradual loss of function, tissue necrosis without inflammation. |
| Key Proteins | Gasdermins, caspase-1, IL-1b, IL-18. | Cathepsins, lysosomal enzymes. |
| Biological Role | Host defense against infection, triggers inflammation. | Response to chronic injury or aging, tissue remodeling. |
| Inflammatory Response | Strong pro-inflammatory response. | Minimal to no inflammation. |
| Cell Death Type | Programmed, regulated cell death. | Degenerative, non-programmed cell death. |
Introduction to Pyroptosis and Necrobiosis
Pyroptosis is a form of programmed cell death characterized by the activation of inflammatory caspases, leading to cell swelling, membrane rupture, and release of pro-inflammatory cytokines like IL-1b. Necrobiosis refers to the physiological degeneration and death of cells, particularly in connective tissues, without inducing inflammation or immune response. Understanding the molecular mechanisms and pathological roles of pyroptosis and necrobiosis is crucial for developing targeted therapies in diseases involving inflammation and tissue remodeling.
Defining Pyroptosis: Mechanisms and Triggers
Pyroptosis is a form of programmed cell death characterized by the activation of inflammasomes and caspase-1, leading to the cleavage of gasdermin D and the formation of membrane pores. This process results in the rapid release of pro-inflammatory cytokines such as IL-1b and IL-18, driving an inflammatory response. Unlike necrobiotic cell death, pyroptosis is primarily triggered by intracellular pathogens and danger-associated molecular patterns, linking it closely to innate immune defense mechanisms.
Understanding Necrobiosis: Biological Process and Causes
Necrobiosis is a natural biological process involving the organized degeneration and death of cells, often seen in connective tissues where cells are gradually replaced without triggering inflammation. Unlike pyroptotic cell death, which is an inflammatory and programmed response to infection or cellular damage involving caspase activation and pore formation, necrobiosis is typically associated with normal cell turnover and tissue remodeling. Common causes of necrobiosis include aging, mechanical stress, and chronic low-level damage, contributing to cellular aging and tissue homeostasis without provoking immune system activation.
Molecular Pathways Involved in Pyroptosis
Pyroptosis is a form of programmed cell death characterized by the activation of inflammatory caspases such as caspase-1, caspase-4, and caspase-5, which cleave gasdermin D to form membrane pores leading to cell lysis and release of pro-inflammatory cytokines IL-1b and IL-18. In contrast, necrobiotic cell death lacks the inflammatory caspase activation and involves distinct signaling pathways such as mitochondrial dysfunction, ROS generation, and lysosomal destabilization leading to cellular breakdown without cytokine release. The inflammasome complex, including NLRP3, plays a central role in pyroptotic molecular pathways, linking pathogen recognition with inflammatory responses essential for innate immunity.
Cellular Changes During Necrobiosis
Necrobiosis involves the gradual, physiologic degeneration of cells characterized by swelling, chromatin condensation, and eventual cell membrane rupture without inflammatory response. Cellular changes include cytoplasmic vacuolization, organelle disintegration, and nuclear pyknosis, distinguishing it from pyroptosis which triggers rapid cell lysis with pro-inflammatory cytokine release. Necrobiotic processes maintain tissue homeostasis and cellular turnover, contrasting with pyroptotic pathways involved in pathogen defense and inflammation.
Key Differences Between Pyroptotic and Necrobiotic Cell Death
Pyroptotic cell death is an inflammatory process characterized by gasdermin-mediated plasma membrane pore formation, leading to cell swelling, lysis, and the release of pro-inflammatory cytokines such as IL-1b and IL-18. In contrast, necrobiotic cell death involves a degenerative process marked by coagulative necrosis and the breakdown of necrotic tissue with minimal inflammatory response. Key differences include the presence of inflammasome activation and cytokine release in pyroptosis, whereas necrobiosis is defined by features like collagen degradation and tissue remodeling without direct cytokine involvement.
Clinical Implications of Pyroptosis and Necrobiosis
Pyroptosis is a form of programmed cell death characterized by inflammasome activation and release of pro-inflammatory cytokines, which plays a critical role in controlling infections and modulating inflammatory diseases such as sepsis and autoimmune disorders. Necrobiosis involves the degeneration and death of cells often associated with chronic inflammation and tissue remodeling, commonly observed in conditions like granulomatous diseases and necrobiosis lipoidica. Understanding the distinct mechanisms and clinical implications of pyroptosis and necrobiosis aids in developing targeted therapies for inflammatory and degenerative pathologies.
Diagnostic Markers for Pyroptotic vs Necrobiotic Cells
Diagnostic markers distinguishing pyroptotic cells include gasdermin D cleavage and caspase-1 activation, leading to pore formation in the plasma membrane and release of pro-inflammatory cytokines like IL-1b and IL-18. Necrobiotic cells, characterized by histological features such as granular eosinophilic cytoplasm and palisading histiocytes, lack these specific biochemical signatures but show increased presence of necrotic debris and altered expression of matrix metalloproteinases. Immunohistochemical detection of cleaved gasdermin D and elevated IL-1b serves as reliable indicators for pyroptosis, while conventional staining methods identify necrobiosis through tissue architecture changes and cellular degeneration patterns.
Therapeutic Strategies Targeting Pyroptosis and Necrobiosis
Therapeutic strategies targeting pyroptosis focus on inhibiting inflammasome activation and gasdermin D cleavage to reduce inflammatory cell death and associated tissue damage. In contrast, necrobiotic therapies aim to modulate cellular stress responses and enhance clearance of necrobiotic cells to prevent chronic inflammation and fibrosis. Emerging drug candidates include caspase-1 inhibitors for pyroptosis and antioxidants or autophagy modulators for necrobiosis, highlighting distinct molecular pathways for tailored treatments.
Future Directions in Pyroptotic and Necrobiotic Research
Future directions in pyroptotic and necrobiotic research emphasize elucidating molecular pathways and identifying specific biomarkers to distinguish these forms of cell death. Advanced techniques like single-cell sequencing and real-time imaging are poised to enhance understanding of their roles in inflammation and tissue homeostasis. Translational studies aim to develop targeted therapeutics that modulate pyroptosis and necrobiosis to treat inflammatory diseases and cancer.
Pyroptotic Infographic
 libterm.com
libterm.com