Combustion is a chemical process where a fuel reacts rapidly with oxygen, producing heat and light. This exothermic reaction is fundamental to engines, heating systems, and power generation, influencing efficiency and emissions. Discover how understanding combustion can improve your energy use by exploring the insights in this article.
Table of Comparison
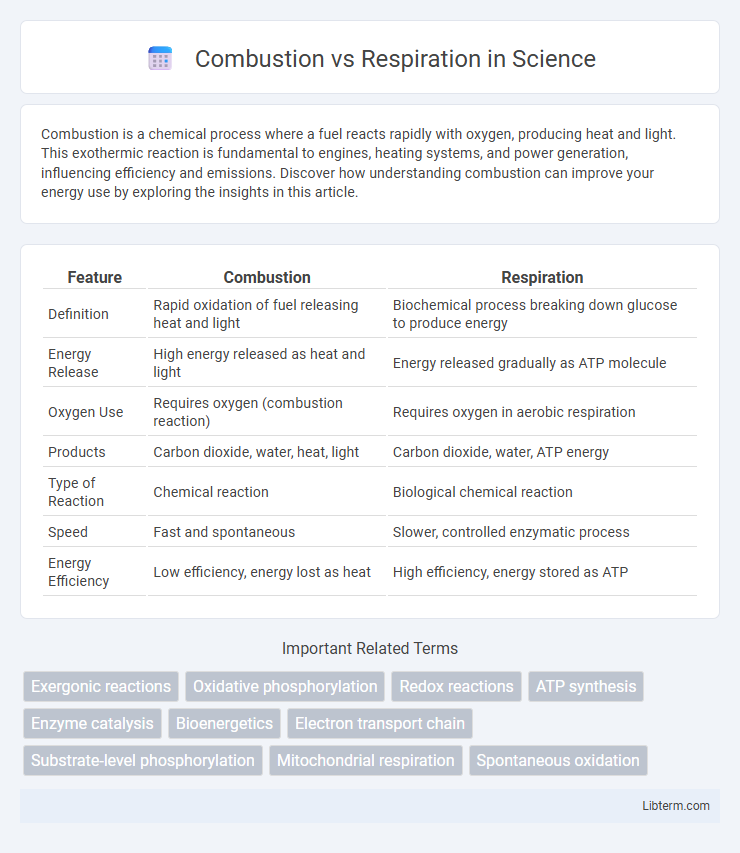
| Feature | Combustion | Respiration |
|---|---|---|
| Definition | Rapid oxidation of fuel releasing heat and light | Biochemical process breaking down glucose to produce energy |
| Energy Release | High energy released as heat and light | Energy released gradually as ATP molecule |
| Oxygen Use | Requires oxygen (combustion reaction) | Requires oxygen in aerobic respiration |
| Products | Carbon dioxide, water, heat, light | Carbon dioxide, water, ATP energy |
| Type of Reaction | Chemical reaction | Biological chemical reaction |
| Speed | Fast and spontaneous | Slower, controlled enzymatic process |
| Energy Efficiency | Low efficiency, energy lost as heat | High efficiency, energy stored as ATP |
Introduction to Combustion and Respiration
Combustion is a chemical process where a substance reacts rapidly with oxygen, producing heat and light, typically involving hydrocarbons and releasing carbon dioxide and water. Respiration is a biological process in cells where glucose molecules are broken down with oxygen to generate energy in the form of ATP, along with the release of carbon dioxide and water. Both processes involve oxidation reactions but differ in complexity, purpose, and byproducts within living organisms versus inanimate materials.
Defining Combustion: Key Characteristics
Combustion is a rapid exothermic chemical reaction between a fuel and an oxidant, typically oxygen, producing heat, light, carbon dioxide, and water vapor. Key characteristics include the release of energy in the form of flames, the requirement of an ignition source to initiate the reaction, and the formation of combustion products such as carbon monoxide and nitrogen oxides under incomplete combustion conditions. Unlike cellular respiration, combustion occurs externally and involves the complete oxidation of fuels like hydrocarbons in an open environment.
Understanding Respiration: Biological Overview
Respiration is a biochemical process in living organisms where glucose molecules are broken down with oxygen to release energy in the form of ATP, essential for cellular functions. Unlike combustion, which is a rapid oxidation reaction producing heat and light, cellular respiration is a controlled, enzyme-catalyzed sequence occurring in mitochondria. This biological pathway includes glycolysis, the Krebs cycle, and the electron transport chain, facilitating efficient energy extraction from nutrients.
Chemical Equations: Combustion vs Respiration
The chemical equation for combustion typically involves the rapid oxidation of a hydrocarbon fuel, such as octane (C8H18), reacting with oxygen (O2) to produce carbon dioxide (CO2), water (H2O), and energy: C8H18 + 12.5O2 - 8CO2 + 9H2O + energy. Cellular respiration follows a similar but controlled reaction where glucose (C6H12O6) combines with oxygen to yield carbon dioxide, water, and adenosine triphosphate (ATP), represented as C6H12O6 + 6O2 - 6CO2 + 6H2O + energy (ATP). Both processes involve oxidation and energy release, but combustion is a rapid, uncontrolled reaction, while respiration is enzymatically regulated for biological energy production.
Energy Release and Utilization in Both Processes
Combustion releases energy rapidly as heat through the oxidation of fuels, often resulting in flame and light, whereas respiration releases energy gradually in cells by enzymatically breaking down glucose to produce ATP. Energy from combustion is mostly lost as heat, making it largely unusable for biological functions, while respiration efficiently captures energy in chemical bonds for cellular activities. The controlled nature of respiration allows organisms to harness energy for metabolism, growth, and repair, contrasting with the uncontrolled energy dissipation in combustion.
Oxygen’s Role in Combustion and Respiration
Oxygen acts as a crucial oxidizing agent in both combustion and respiration, facilitating the release of energy through chemical reactions. In combustion, oxygen rapidly reacts with fuel to produce heat, light, carbon dioxide, and water. In cellular respiration, oxygen accepts electrons at the mitochondrial electron transport chain, enabling efficient ATP production by oxidizing glucose molecules.
Byproducts: Comparing Output of Both Reactions
Combustion produces byproducts such as carbon dioxide, water vapor, and often harmful pollutants including carbon monoxide and nitrogen oxides, depending on fuel type and combustion conditions. Cellular respiration generates carbon dioxide and water as byproducts through the metabolic breakdown of glucose in the presence of oxygen, releasing energy stored as ATP. Unlike combustion, respiration is a controlled biochemical process that minimizes harmful emissions while sustaining life processes.
Environmental Impact and Relevance
Combustion releases significant amounts of carbon dioxide and pollutants such as nitrogen oxides and particulate matter, contributing heavily to air pollution and climate change. Respiration, a natural biological process, emits much smaller quantities of carbon dioxide, playing a crucial role in the carbon cycle without causing environmental harm. Understanding the environmental impact of combustion versus respiration highlights the importance of reducing fossil fuel combustion to mitigate global warming and improve air quality.
Practical Applications in Daily Life
Combustion powers vehicles and industrial machines by converting fuel into energy through rapid oxidation, driving transportation and manufacturing processes. Respiration enables cellular energy production in living organisms by breaking down glucose and oxygen, sustaining bodily functions and physical activity. Understanding these processes improves energy efficiency in engines and promotes health through optimized metabolic functions.
Summary: Key Differences and Similarities
Combustion and respiration both involve the oxidation of fuels to release energy, with combustion occurring rapidly at high temperatures and respiration occurring gradually at controlled physiological temperatures. Combustion produces heat and light, primarily releasing carbon dioxide and water, while respiration converts glucose and oxygen into energy (ATP), carbon dioxide, and water within living cells. Both processes require oxygen and generate carbon dioxide and water as byproducts, but respiration is a biochemical process essential for cellular metabolism, whereas combustion is a chemical process used for energy generation outside biological systems.
Combustion Infographic
 libterm.com
libterm.com