Quantum mechanics explores the behavior of particles at the smallest scales, revealing phenomena like superposition and entanglement that challenge classical physics. This field underpins emerging technologies such as quantum computing and secure quantum communication, promising revolutionary advancements in processing power and information security. Discover how quantum principles are reshaping science and technology in the rest of this article.
Table of Comparison
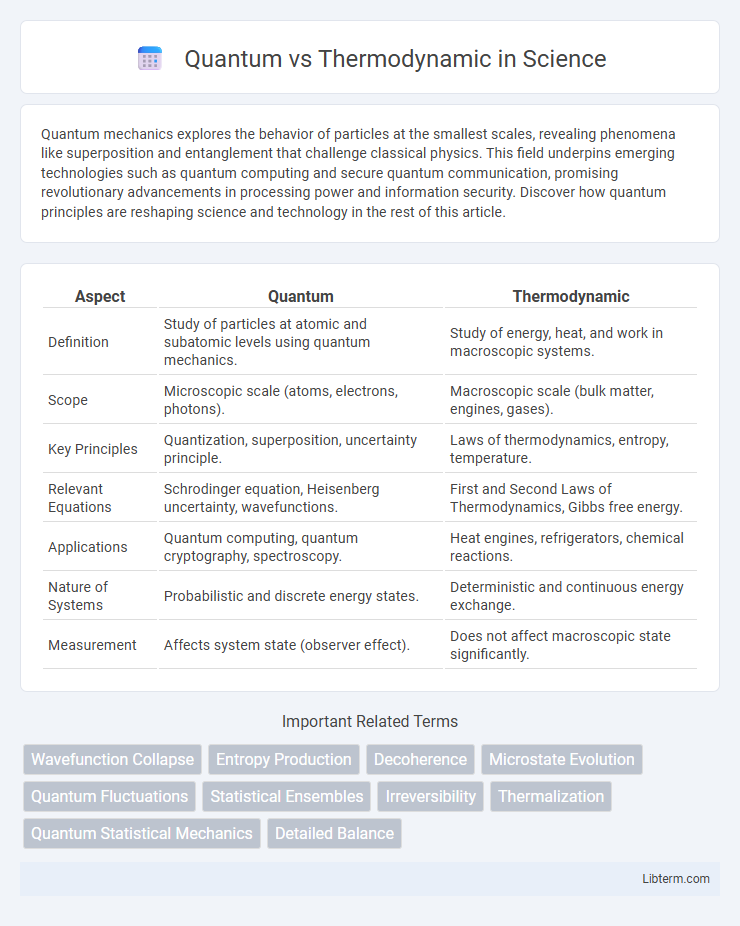
| Aspect | Quantum | Thermodynamic |
|---|---|---|
| Definition | Study of particles at atomic and subatomic levels using quantum mechanics. | Study of energy, heat, and work in macroscopic systems. |
| Scope | Microscopic scale (atoms, electrons, photons). | Macroscopic scale (bulk matter, engines, gases). |
| Key Principles | Quantization, superposition, uncertainty principle. | Laws of thermodynamics, entropy, temperature. |
| Relevant Equations | Schrodinger equation, Heisenberg uncertainty, wavefunctions. | First and Second Laws of Thermodynamics, Gibbs free energy. |
| Applications | Quantum computing, quantum cryptography, spectroscopy. | Heat engines, refrigerators, chemical reactions. |
| Nature of Systems | Probabilistic and discrete energy states. | Deterministic and continuous energy exchange. |
| Measurement | Affects system state (observer effect). | Does not affect macroscopic state significantly. |
Introduction to Quantum and Thermodynamic Principles
Quantum principles govern the behavior of particles at microscopic scales, emphasizing wave-particle duality, quantization of energy levels, and probability amplitudes. Thermodynamic principles describe macroscopic systems through laws involving energy transfer, entropy, temperature, and equilibrium states. The foundational difference lies in quantum mechanics addressing discrete energy states and probabilistic outcomes, while thermodynamics focuses on bulk properties and irreversible processes driven by entropy changes.
Defining Quantum Mechanics: Core Concepts
Quantum mechanics explores the behavior of particles at atomic and subatomic scales, where wave-particle duality and quantum superposition define state probabilities rather than deterministic positions. Central concepts include the quantization of energy, wavefunctions derived from Schrodinger's equation, and the uncertainty principle limiting simultaneous knowledge of certain properties. Unlike thermodynamics, which addresses macroscopic energy exchange and entropy, quantum mechanics examines discrete phenomena influencing fundamental particles and their interactions.
Fundamentals of Thermodynamics Explained
Thermodynamics is the study of energy transfer and the laws governing heat, work, and entropy in macroscopic systems, rooted in classical physics. Quantum mechanics explains the behavior of particles at atomic and subatomic levels, providing a microscopic foundation for thermodynamic properties through quantum states and statistical mechanics. The fundamentals of thermodynamics revolve around its four laws, which describe energy conservation, entropy increase, absolute zero temperature, and the relationship between thermodynamic variables.
Key Differences Between Quantum and Thermodynamic Systems
Quantum systems operate at the microscopic scale, governed by principles such as superposition, entanglement, and discrete energy levels, whereas thermodynamic systems describe macroscopic behaviors based on bulk properties like temperature, pressure, and entropy. Quantum mechanics deals with wavefunctions and probability amplitudes, while thermodynamics relies on laws like the first and second laws to explain energy transformations and equilibrium states. Quantum effects become negligible in thermodynamic limits where statistical ensembles and classical approximations accurately describe system behavior.
Quantum States vs Thermodynamic States
Quantum states describe the precise configuration of particles at the microscopic level, characterized by wavefunctions and governed by the principles of quantum mechanics. Thermodynamic states represent macroscopic properties such as temperature, pressure, and volume, summarizing the collective behavior of vast numbers of particles using state variables and thermodynamic potentials. The fundamental difference lies in quantum states capturing individual particle probabilities, while thermodynamic states express statistical averages of large ensembles.
Energy Transfer: Quantum vs Classical Perspectives
Energy transfer in quantum systems occurs through discrete quanta such as photons or phonons, governed by principles of superposition and entanglement, enabling phenomena like tunneling and coherence. In contrast, classical thermodynamics describes energy transfer macroscopically via heat conduction, convection, and radiation, relying on statistical averages and continuous energy flow. Quantum energy transfer often exhibits non-classical effects that cannot be fully explained by classical thermodynamic laws, highlighting fundamental differences between microscopic quantum processes and macroscopic thermal behavior.
Role of Probability in Quantum Mechanics and Thermodynamics
In quantum mechanics, probability governs the behavior of particles through wavefunctions, where measurement outcomes are inherently probabilistic and described by the Born rule. Thermodynamics uses probability to describe macroscopic properties emerging from the statistical behavior of vast ensembles of particles, relying on concepts like entropy and the Boltzmann distribution. Both fields employ probability to bridge microstates and observable phenomena, but quantum mechanics focuses on fundamental uncertainty, whereas thermodynamics addresses statistical distributions over many particles.
Entropy: A Bridge Between Quantum and Thermodynamic Theories
Entropy serves as a fundamental concept linking quantum mechanics and thermodynamics by quantifying disorder and information within physical systems. In quantum theory, entropy is characterized by the von Neumann entropy, which measures the uncertainty of a quantum state's density matrix. Thermodynamic entropy, defined by the Boltzmann equation, correlates with macroscopic irreversibility and energy dispersal, establishing a crucial connection between microscopic quantum phenomena and classical thermodynamic behavior.
Practical Applications in Technology and Industry
Quantum mechanics underpins the development of quantum computing, enabling unprecedented processing speeds and enhanced cryptographic security in communication networks. Thermodynamics drives advancements in energy efficiency, powering innovations in heat engines, refrigeration systems, and sustainable industrial processes. Both fields contribute to cutting-edge technologies: quantum sensors improve precision measurements, while thermodynamic principles optimize large-scale manufacturing and energy management systems.
Future Directions: Integrating Quantum and Thermodynamic Approaches
Future directions in scientific research emphasize integrating quantum mechanics with thermodynamic principles to enhance our understanding of energy transfer and entropy at the nanoscale. Developing quantum thermodynamic frameworks aims to optimize quantum computing efficiency and improve nanoscale heat engines by leveraging quantum coherence and entanglement. Advancements in this integration could revolutionize energy technologies, enabling precise control over quantum states while adhering to thermodynamic laws.
Quantum Infographic
 libterm.com
libterm.com