Isobaric processes occur at constant pressure, making them essential in thermodynamics and various engineering applications. Understanding how volume and temperature change during these processes can improve your grasp of energy transfer and system behavior. Explore the rest of the article to learn more about isobaric transformations and their practical implications.
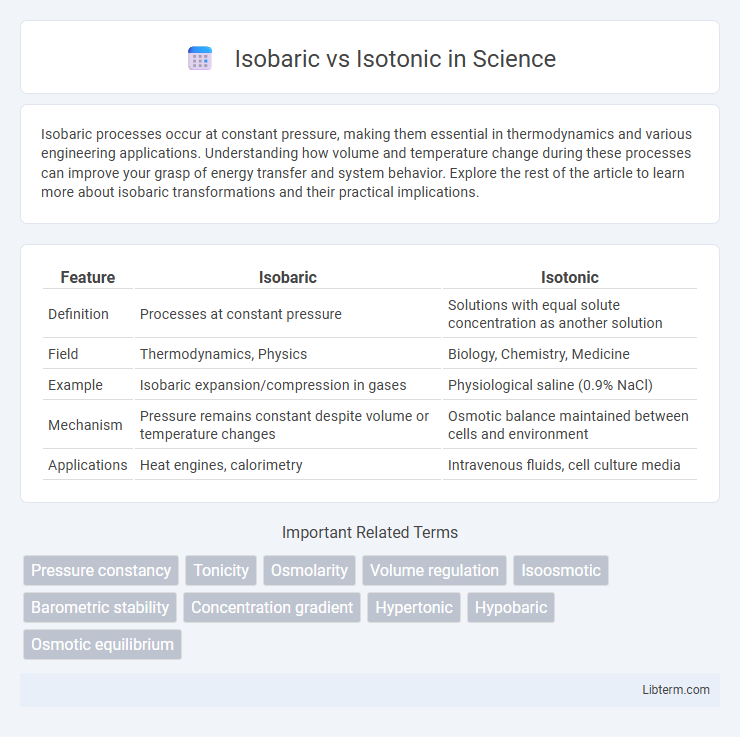
Table of Comparison
| Feature | Isobaric | Isotonic |
|---|---|---|
| Definition | Processes at constant pressure | Solutions with equal solute concentration as another solution |
| Field | Thermodynamics, Physics | Biology, Chemistry, Medicine |
| Example | Isobaric expansion/compression in gases | Physiological saline (0.9% NaCl) |
| Mechanism | Pressure remains constant despite volume or temperature changes | Osmotic balance maintained between cells and environment |
| Applications | Heat engines, calorimetry | Intravenous fluids, cell culture media |
Introduction to Isobaric and Isotonic Concepts
Isobaric processes occur at constant pressure, commonly observed in thermodynamics when volume and temperature vary but pressure remains unchanged. Isotonic solutions, crucial in biology and medicine, maintain equal osmotic pressure across cell membranes, preventing cellular swelling or shrinkage. Understanding these concepts helps in analyzing physical systems under steady pressure and designing medical treatments involving fluid balance.
Definitions: Isobaric vs Isotonic
Isobaric refers to processes or conditions occurring at constant pressure, commonly studied in thermodynamics and physiology to describe pressure-maintaining states. Isotonic describes solutions or exercises where solute concentration matches that of cells, preventing net water movement across membranes or indicating muscle contraction with consistent tension. Understanding isobaric and isotonic definitions is essential in fields like exercise science, biology, and physical chemistry for proper application and analysis.
Key Differences Between Isobaric and Isotonic Processes
Isobaric processes maintain constant pressure while allowing volume and temperature to change, commonly observed in heating or cooling at atmospheric pressure. Isotonic processes, primarily related to biological systems, maintain constant solute concentration to prevent osmotic imbalance, crucial for cell fluid regulation. The key difference lies in isobaric referring to pressure constancy in thermodynamics, whereas isotonic pertains to solute concentration and osmotic pressure balance in physiology.
Isobaric Processes Explained
Isobaric processes occur at a constant pressure, meaning the system's pressure remains unchanged while volume and temperature may vary. In thermodynamics, this type of process is significant for understanding heat transfer, as the work done by or on the system equals the pressure multiplied by the change in volume (W = PDV). Isobaric conditions are common in many engineering applications, such as pistons in internal combustion engines and phase changes in liquids.
Isotonic Processes Explained
Isotonic processes involve changes in volume while maintaining constant pressure, typically seen in physiological settings like muscle contractions where muscle length changes but tension remains steady. These processes differ from isobaric ones, where pressure remains constant but volume and temperature can vary. Understanding isotonic conditions is crucial for studying cellular mechanics and fluid balance in biological systems.
Real-World Examples of Isobaric and Isotonic Changes
Isobaric changes occur in weather systems where atmospheric pressure remains constant while temperature varies, such as during the formation of clouds in a constant-pressure front. Isotonic changes are seen in biological systems, like red blood cells immersed in saline solutions matching their internal ion concentration, preventing cell swelling or shrinking. Industrial processes involving fermentation or chemical reactions often utilize isobaric conditions to control temperature without altering pressure, ensuring consistent product quality.
Applications in Science and Engineering
Isobaric processes, characterized by constant pressure, are crucial in thermodynamics for designing engines and understanding chemical reactions in confined systems. Isotonic conditions, where solute concentration remains constant, are extensively applied in biomedical engineering to maintain cell viability during tissue culture and intravenous therapies. Both concepts underpin experimental protocols and industrial practices, optimizing reaction conditions and physiological environments.
Importance in Thermodynamics and Physiology
Isobaric and isotonic processes play crucial roles in both thermodynamics and physiology by maintaining constant pressure and osmotic balance, respectively. In thermodynamics, isobaric processes facilitate heat exchange at steady pressure, essential for engine cycles and metabolic heat management. Physiologically, isotonic conditions preserve cell volume and function by equalizing solute concentration inside and outside cells, preventing osmotic stress and ensuring homeostasis.
Comparative Table: Isobaric vs Isotonic
Isobaric processes maintain constant pressure throughout the reaction, while isotonic conditions refer to equal solute concentrations across cell membranes, crucial in biological contexts. Isobaric changes involve volume and temperature adjustments to keep pressure steady, whereas isotonic solutions prevent net water movement, preserving cell volume. Both concepts play vital roles in thermodynamics and physiology, respectively, highlighting distinct mechanisms of equilibrium maintenance.
Conclusion: Choosing Between Isobaric and Isotonic
Selecting between isobaric and isotonic techniques depends on the specific application requirements, such as pressure stability or osmotic balance. Isobaric methods are ideal for processes needing constant pressure, while isotonic approaches suit scenarios requiring equal solute concentration to prevent cellular damage. Understanding the context of usage enables optimal choice, improving efficiency and outcomes in clinical or experimental settings.
Isobaric Infographic
 libterm.com
libterm.com