Alkaliphiles are microorganisms that thrive in highly alkaline environments, often with pH levels above 9. These extremophiles possess unique adaptations allowing their cellular processes to function optimally under such conditions, making them of great interest in industrial biotechnology. Discover how alkaliphiles can influence various applications and what role they may play in your scientific explorations by reading the full article.
Table of Comparison
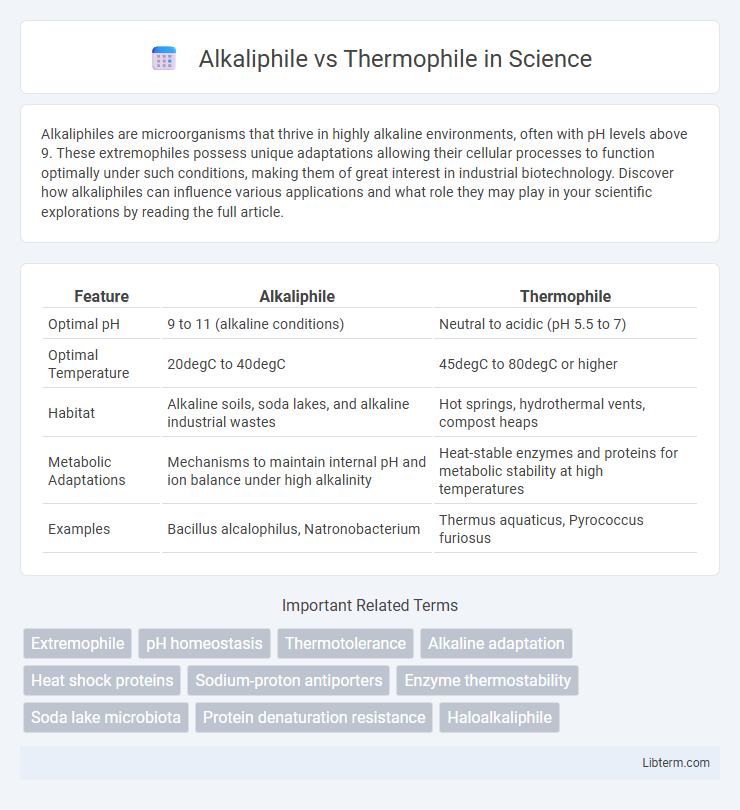
| Feature | Alkaliphile | Thermophile |
|---|---|---|
| Optimal pH | 9 to 11 (alkaline conditions) | Neutral to acidic (pH 5.5 to 7) |
| Optimal Temperature | 20degC to 40degC | 45degC to 80degC or higher |
| Habitat | Alkaline soils, soda lakes, and alkaline industrial wastes | Hot springs, hydrothermal vents, compost heaps |
| Metabolic Adaptations | Mechanisms to maintain internal pH and ion balance under high alkalinity | Heat-stable enzymes and proteins for metabolic stability at high temperatures |
| Examples | Bacillus alcalophilus, Natronobacterium | Thermus aquaticus, Pyrococcus furiosus |
Introduction to Extremophiles
Extremophiles are microorganisms that thrive in conditions considered hostile for most life forms, such as high temperature or extreme pH levels. Alkaliphiles specifically adapt to environments with high pH values, often above 9, enabling them to survive in alkaline soils, lakes, and industrial wastes. Thermophiles, on the other hand, flourish at elevated temperatures, typically between 45degC and 122degC, commonly found in hot springs, hydrothermal vents, and compost heaps.
Defining Alkaliphiles
Alkaliphiles are extremophiles that thrive in highly alkaline environments with pH levels typically above 9, often found in soda lakes and alkaline soils. These organisms possess specialized adaptations like unique cell membrane structures and enzymes that remain stable and functional in high pH conditions. In contrast, thermophiles are heat-loving microbes that prosper at elevated temperatures, usually between 45degC and 80degC, highlighting their distinct ecological niches and physiological mechanisms.
Defining Thermophiles
Thermophiles are microorganisms that thrive at high temperatures, typically between 41degC and 122degC, found in environments such as hot springs and hydrothermal vents. Alkaliphiles, in contrast, prefer highly alkaline environments with pH levels above 9, often inhabiting soda lakes and alkaline soils. Understanding thermophiles' ability to maintain protein stability and enzymatic function at elevated temperatures highlights their significance in industrial applications like biotechnology and biofuel production.
Environmental Habitats of Alkaliphiles
Alkaliphiles predominantly thrive in high pH environments such as soda lakes, alkaline soils, and industrial wastewaters where pH values often exceed 9. These microorganisms have adapted to sustain growth in environments characterized by elevated alkalinity, which is inhospitable to most other life forms. In contrast, thermophiles inhabit extreme heat environments like hot springs and hydrothermal vents, with optimal growth temperatures ranging from 45degC to 80degC or higher.
Environmental Habitats of Thermophiles
Thermophiles thrive in extreme high-temperature environments such as hot springs, deep-sea hydrothermal vents, and geothermal soils where temperatures range from 45degC to 122degC. These microorganisms have specialized enzymes and cellular structures that allow them to maintain stability and metabolic function under intense heat conditions. Unlike alkaliphiles that prefer high pH environments, thermophiles are primarily adapted to thermal extremes, making their habitats critical for understanding heat-resistant biological processes.
Molecular Adaptations in Alkaliphiles
Alkaliphiles exhibit specialized molecular adaptations such as robust cell wall structures with high acidic polymer content to maintain cellular integrity in high pH environments, contrasting thermophiles that rely on heat-stable enzymes and protein folding mechanisms to withstand extreme temperatures. Alkaliphilic enzymes possess unique amino acid substitutions that enhance stability and function under alkaline conditions by optimizing ion transport and intracellular pH homeostasis. These adaptations enable alkaliphiles to thrive in environments with pH values typically above 9, demonstrating distinct molecular strategies from thermophiles, which are adapted to survive at temperatures often exceeding 60degC.
Molecular Adaptations in Thermophiles
Thermophiles exhibit molecular adaptations such as increased thermostability of proteins and enzymes through enhanced hydrogen bonding, salt bridges, and hydrophobic interactions, enabling functionality at high temperatures. Their membrane lipids contain ether linkages and saturated fatty acids that maintain membrane integrity in extreme heat. DNA stabilizing proteins and reverse gyrase also play crucial roles in protecting genetic material from thermal denaturation in thermophilic organisms.
Industrial Applications of Alkaliphiles
Alkaliphiles thrive in high pH environments and are widely utilized in industries for their production of alkaline-stable enzymes such as proteases, lipases, and cellulases, which are essential in detergents, textile processing, and bioremediation. Their robustness in extreme alkaline conditions enables efficient waste treatment processes and biofuel production, outperforming thermophiles that primarily excel in high-temperature industrial applications. Alkaliphile-derived biocatalysts contribute to environmentally sustainable manufacturing by reducing the need for harsh chemicals and energy-intensive steps.
Industrial Applications of Thermophiles
Thermophiles thrive at high temperatures typically between 45degC and 80degC, making their enzymes highly stable and efficient for industrial applications such as biofuel production, waste management, and pharmaceutical synthesis. These heat-resistant enzymes, including thermostable DNA polymerases and proteases, enable processes to occur faster and with greater yield under extreme conditions that would denature mesophilic enzymes. In contrast, alkaliphiles, which prefer high pH environments, are less commonly exploited in industry compared to thermophiles but are important in applications requiring alkaline tolerance.
Key Differences Between Alkaliphiles and Thermophiles
Alkaliphiles thrive in high pH environments typically above pH 9, while thermophiles grow optimally at elevated temperatures usually between 45degC and 80degC. Alkaliphiles possess cellular adaptations to maintain internal pH homeostasis despite external alkalinity, whereas thermophiles have heat-stable enzymes and proteins to withstand thermal denaturation. These distinct biological mechanisms reflect their adaptation to extreme alkaline versus high-temperature habitats.
Alkaliphile Infographic
 libterm.com
libterm.com