Isomers are compounds with the same molecular formula but different structural arrangements, leading to distinct physical and chemical properties. Understanding the types of isomers, including structural isomers and stereoisomers, is crucial for applications in chemistry and pharmacology. Explore the rest of the article to discover how isomerism impacts various scientific fields and Your daily life.
Table of Comparison
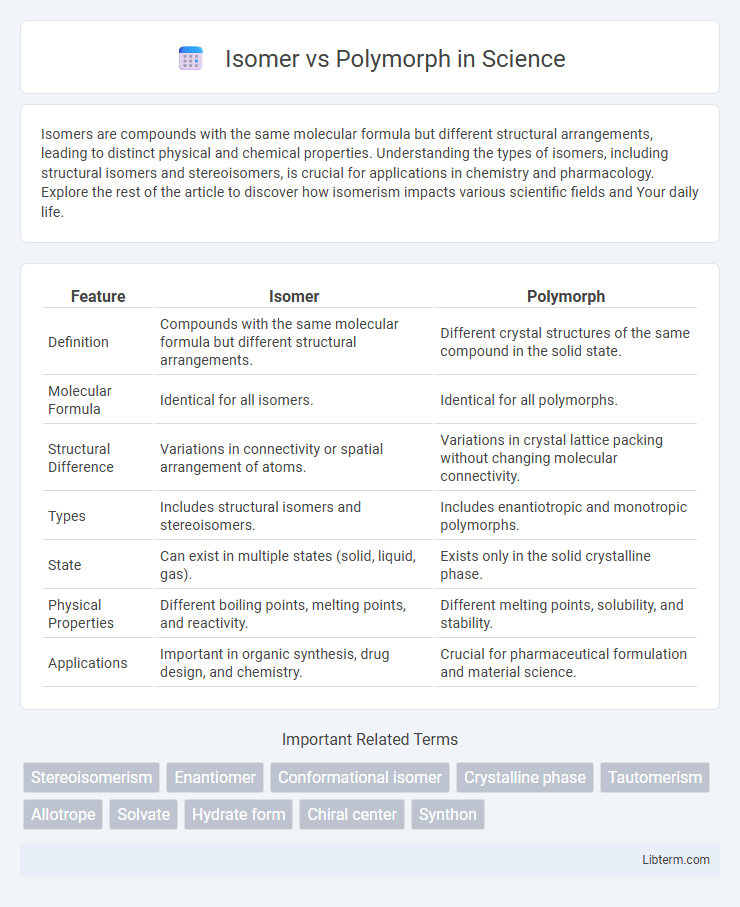
| Feature | Isomer | Polymorph |
|---|---|---|
| Definition | Compounds with the same molecular formula but different structural arrangements. | Different crystal structures of the same compound in the solid state. |
| Molecular Formula | Identical for all isomers. | Identical for all polymorphs. |
| Structural Difference | Variations in connectivity or spatial arrangement of atoms. | Variations in crystal lattice packing without changing molecular connectivity. |
| Types | Includes structural isomers and stereoisomers. | Includes enantiotropic and monotropic polymorphs. |
| State | Can exist in multiple states (solid, liquid, gas). | Exists only in the solid crystalline phase. |
| Physical Properties | Different boiling points, melting points, and reactivity. | Different melting points, solubility, and stability. |
| Applications | Important in organic synthesis, drug design, and chemistry. | Crucial for pharmaceutical formulation and material science. |
Introduction to Isomers and Polymorphs
Isomers are molecules with the same molecular formula but different structural arrangements, resulting in distinct chemical and physical properties. Polymorphs refer to different crystalline forms of the same compound, exhibiting variations in molecular packing and stability. Understanding these differences is crucial in pharmaceuticals, where polymorphs influence drug solubility and bioavailability, while isomers impact pharmacodynamics and efficacy.
Defining Isomers: Concept and Types
Isomers are compounds with the same molecular formula but different arrangements of atoms, leading to distinct properties. They are broadly classified into structural isomers, differing in connectivity of atoms, and stereoisomers, which have the same connectivity but differ in spatial orientation. Understanding the types of isomers--such as chain, positional, and geometric--is crucial for applications in chemistry and pharmaceuticals.
Understanding Polymorphs: Key Characteristics
Polymorphs are distinct crystalline forms of the same chemical compound, differing in molecular arrangement and physical properties such as melting point, solubility, and stability. These variations impact pharmaceutical efficacy, bioavailability, and manufacturing processes, making polymorph characterization critical in drug development. Techniques like X-ray diffraction, differential scanning calorimetry, and spectroscopy are essential for identifying and analyzing polymorphic forms, ensuring consistent product performance and regulatory compliance.
Structural Differences: Isomer vs Polymorph
Isomers are molecules with the same molecular formula but different atomic connectivity, leading to distinct chemical structures and properties. Polymorphs, in contrast, are different crystal forms of the same compound, exhibiting variations only in molecular packing and lattice arrangement without altering the molecular connectivity. Structural differences in isomers involve bond rearrangement, while polymorphs differ solely in their solid-state crystalline organization.
Chemical vs Physical Properties
Isomers differ in chemical properties because they have the same molecular formula but different structural arrangements, affecting reactivity and interactions at the molecular level. Polymorphs exhibit variations in physical properties like melting point, solubility, and crystal structure without altering chemical composition. Understanding these distinctions is crucial in pharmaceutical development, where isomerism influences drug efficacy and polymorphism impacts bioavailability and stability.
Examples of Isomers in Chemistry
Isomers in chemistry refer to compounds with the same molecular formula but different structural arrangements, such as glucose and fructose, which are both C6H12O6 but differ in their atomic connectivity. Structural isomers include butane and isobutane, both C4H10, showcasing different carbon chain arrangements, while stereoisomers like cis- and trans-2-butene vary in spatial orientation around a double bond. These examples highlight the diversity of isomers, impacting properties like boiling points, reactivity, and biological activity.
Common Polymorphs in Pharmaceuticals
Common polymorphs in pharmaceuticals include forms like ritonavir, carbamazepine, and paracetamol, which exhibit distinct crystal structures affecting solubility and bioavailability. Isomers differ in molecular connectivity or spatial arrangement but share the same molecular formula; polymorphs possess identical molecular formulas and connectivity but vary in crystal lattice arrangement. Understanding polymorph behavior is crucial in drug formulation to ensure stability, efficacy, and patent protection.
Analytical Techniques for Identification
Isomers and polymorphs require distinct analytical techniques for accurate identification, leveraging methods like nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) to differentiate isomers based on molecular structure and mass. Polymorphs are primarily distinguished using powder X-ray diffraction (PXRD) and differential scanning calorimetry (DSC), which reveal unique crystal lattice arrangements and thermal behaviors. Raman spectroscopy and infrared (IR) spectroscopy also provide complementary molecular vibrational profiles critical for characterizing both isomeric and polymorphic forms.
Industrial and Biological Significance
Isomers exhibit distinct molecular structures with identical molecular formulas, affecting pharmacological activity and chemical properties crucial for drug design and biochemical pathways. Polymorphs, defined by different crystal structures of the same compound, influence solubility, bioavailability, and stability vital for pharmaceuticals and materials science. Understanding isomerism and polymorphism enables optimization of industrial processes and enhances biological efficacy in drug development and manufacturing.
Summary and Key Takeaways
Isomers are compounds with the same molecular formula but different structural arrangements, while polymorphs are different crystal forms of the same compound exhibiting distinct physical properties. Understanding these differences is crucial in pharmaceuticals, where isomerism affects chemical behavior and polymorphism impacts drug stability and bioavailability. Key takeaways include the significance of isomerism in molecular function and polymorphism in solid-state properties influencing drug formulation and performance.
Isomer Infographic
 libterm.com
libterm.com