Resonance structures illustrate different possible arrangements of electrons within a molecule, showing how electrons can be delocalized to stabilize the compound. Understanding these structures allows you to predict molecule behavior and reactivity more accurately. Explore the rest of the article to deepen your knowledge of resonance and its impact on chemical bonding.
Table of Comparison
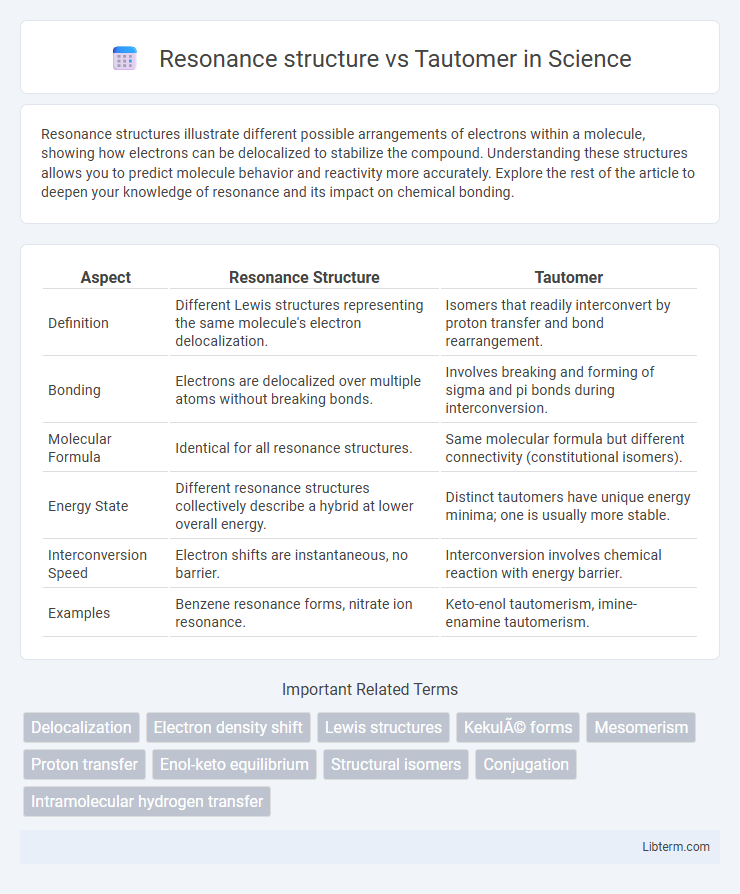
| Aspect | Resonance Structure | Tautomer |
|---|---|---|
| Definition | Different Lewis structures representing the same molecule's electron delocalization. | Isomers that readily interconvert by proton transfer and bond rearrangement. |
| Bonding | Electrons are delocalized over multiple atoms without breaking bonds. | Involves breaking and forming of sigma and pi bonds during interconversion. |
| Molecular Formula | Identical for all resonance structures. | Same molecular formula but different connectivity (constitutional isomers). |
| Energy State | Different resonance structures collectively describe a hybrid at lower overall energy. | Distinct tautomers have unique energy minima; one is usually more stable. |
| Interconversion Speed | Electron shifts are instantaneous, no barrier. | Interconversion involves chemical reaction with energy barrier. |
| Examples | Benzene resonance forms, nitrate ion resonance. | Keto-enol tautomerism, imine-enamine tautomerism. |
Introduction to Resonance Structures and Tautomers
Resonance structures are different Lewis structures representing the same molecule, illustrating the delocalization of electrons within a fixed atomic framework without altering atom connectivity. Tautomers are isomers that differ in the position of protons and electrons, typically involving the relocation of a hydrogen atom and a shift between single and double bonds, resulting in distinct chemical species. Resonance structures depict electron distribution in a single molecule, while tautomers represent discrete molecules in rapid equilibrium.
Fundamental Definitions
Resonance structures are different Lewis structures for the same molecule that represent delocalization of electrons without changing the positions of atoms, maintaining overall molecular identity. Tautomers are isomers that differ by the position of protons and electrons, involving actual structural rearrangement and existing in dynamic equilibrium. Resonance explains electronic distribution within a single species, while tautomerism describes interconversion between distinct chemical entities.
Electronic Delocalization in Resonance
Resonance structures represent different ways of arranging electrons within a single molecule without altering the positions of atoms, emphasizing electronic delocalization to stabilize the molecule by distributing electron density over multiple atoms. Tautomers are distinct isomers formed by the relocation of protons and electrons, resulting in different connectivity and chemical properties. The key difference lies in resonance involving delocalized electrons within a fixed atomic framework, whereas tautomerism involves dynamic structural changes between isomers.
Proton Shifts in Tautomerism
Proton shifts in tautomerism involve the relocation of a proton accompanied by the rearrangement of bonding electrons, resulting in distinct isomers called tautomers that differ in connectivity and chemical properties. Resonance structures, however, depict delocalization of electrons within the same molecular framework without shifting atoms or protons, representing resonance hybrids rather than separate species. Tautomerism typically involves keto-enol or imine-enamine pairs, where proton transfer leads to dynamic equilibrium between discrete forms, crucial for biochemical processes and reaction mechanisms.
Key Differences: Resonance vs Tautomerism
Resonance structures represent different electron arrangements within the same molecule, with atoms retaining their connectivity and only electron positions shifting to depict delocalized bonding. Tautomers are distinct isomers differing in the position of atoms, typically involving proton transfer and resulting in different chemical species with unique connectivity and properties. Key differences lie in resonance being a theoretical construct illustrating electron delocalization without molecular change, whereas tautomerism involves actual chemical equilibria between structurally different isomers.
Common Examples in Organic Chemistry
Resonance structures are different Lewis structures of the same molecule showing delocalized electrons, commonly seen in benzene and nitrate ions where electrons shift positions without changing atom connectivity. Tautomers, such as keto-enol pairs in acetylacetone or aldehyde-alcohol forms in glucose, represent isomers differing in the position of protons and double bonds, interconverting through chemical equilibrium. These concepts are fundamental in organic chemistry for understanding stability, reactivity, and molecular behavior of compounds like phenol, pyridine, and b-dicarbonyl compounds.
Structural Representation and Notation
Resonance structures represent different Lewis structures of the same molecule where electrons are delocalized, illustrated by double-headed arrows between the forms without changing atom connectivity. Tautomers are isomers differing in atom positions and bonding, typically involving proton shifts, shown with equilibrium arrows to indicate interconversion. Resonance involves hybridization of multiple structures into one resonance hybrid, while tautomerism reflects dynamic chemical equilibrium between distinct structural entities.
Role in Chemical Reactivity and Stability
Resonance structures represent different electron arrangements within a molecule that contribute to delocalizing charge and enhancing stability by lowering the overall energy. Tautomers are isomers differing in proton position and bond connectivity, interconverting rapidly and influencing chemical reactivity by providing multiple reactive sites or forms. Resonance stabilizes molecules by electron delocalization without altering the atomic framework, while tautomerism involves structural rearrangement impacting reaction pathways and equilibrium compositions.
Significance in Biological Systems
Resonance structures depict the delocalization of electrons within a molecule, contributing to the stability and reactivity of biomolecules such as nucleic acids and proteins by allowing electron density to be distributed over multiple atoms. Tautomers are isomers that readily interconvert by the migration of a proton and shift of a double bond, playing a crucial role in genetic mutation and enzyme catalysis due to their ability to alter hydrogen bonding patterns and molecular recognition. Understanding the distinction between resonance structures and tautomers is essential for interpreting molecular behavior in biological systems, influencing drug design, DNA base pairing fidelity, and metabolic pathways.
Summary and Comparative Table
Resonance structures represent different Lewis structures of the same molecule where electron positions vary without changing atomic connectivity, whereas tautomers are distinct isomers differing by the position of a proton and a double bond, allowing chemical interconversion. Resonance contributes to the delocalization of electrons stabilizing a single hybrid structure, while tautomerism involves dynamic equilibrium between isomers with different properties. The comparative table highlights resonance as static electron reorganization within identical bond frameworks, contrasted with tautomerism's reversible proton shift altering bonding patterns and molecular identity.
Resonance structure Infographic
 libterm.com
libterm.com