Orthoclase and microcline are both potassium feldspars commonly found in igneous and metamorphic rocks, distinguished mainly by their crystal structures and formation conditions. Orthoclase typically crystallizes at higher temperatures and exhibits a monoclinic structure, while microcline forms at lower temperatures with a triclinic crystal system and shows characteristic cross-hatch twinning patterns. Explore the rest of the article to understand their geological significance and how these minerals impact your rock identification and analysis.
Table of Comparison
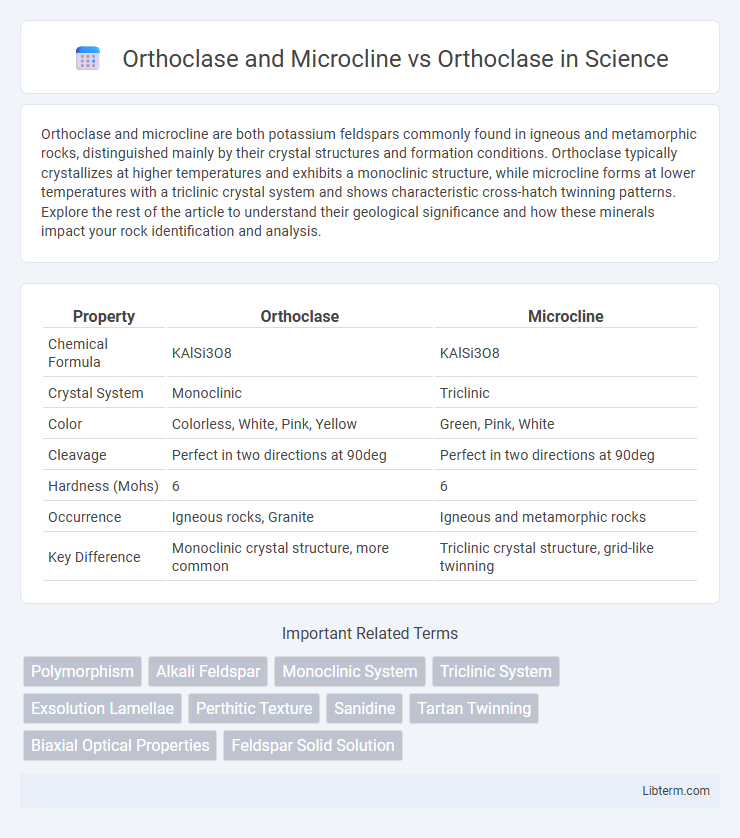
| Property | Orthoclase | Microcline |
|---|---|---|
| Chemical Formula | KAlSi3O8 | KAlSi3O8 |
| Crystal System | Monoclinic | Triclinic |
| Color | Colorless, White, Pink, Yellow | Green, Pink, White |
| Cleavage | Perfect in two directions at 90deg | Perfect in two directions at 90deg |
| Hardness (Mohs) | 6 | 6 |
| Occurrence | Igneous rocks, Granite | Igneous and metamorphic rocks |
| Key Difference | Monoclinic crystal structure, more common | Triclinic crystal structure, grid-like twinning |
Introduction to Orthoclase and Microcline
Orthoclase and Microcline are both feldspar minerals belonging to the potassium feldspar group, sharing the chemical formula KAlSi3O8 but differing in their crystal structures. Orthoclase typically forms monoclinic crystals, while Microcline has a triclinic crystal system, giving it distinct physical properties such as cleavage and twinning patterns. These structural differences influence their identification in geological samples and determine their applications in ceramics, glass-making, and as gemstones.
Chemical Composition: Orthoclase vs Microcline
Orthoclase and microcline share the chemical composition KAlSi3O8, both classified as potassium feldspar minerals, yet they differ in crystal structure and formation temperature. Orthoclase typically forms in high-temperature igneous environments, exhibiting a monoclinic crystal system, while microcline crystallizes at lower temperatures with a triclinic system, often showing characteristic grid-like twinning. These structural differences influence their physical properties and stability, despite identical chemical formulas.
Crystal Structure Differences
Orthoclase and microcline are both potassium feldspars with similar chemical compositions but distinct crystal structures; orthoclase crystallizes in the monoclinic system, while microcline forms in the triclinic system. This difference in crystal symmetry affects their physical properties and the arrangement of aluminum and silicon atoms within their tetrahedral frameworks. Microcline's triclinic structure leads to distinctive grid-like cross-hatch twinning patterns visible under a microscope, which is absent in orthoclase's monoclinic crystals.
Physical Properties Comparison
Orthoclase and Microcline, both potassium feldspars, exhibit distinct physical properties despite their similar chemical compositions. Orthoclase typically shows a monoclinic crystal system with two cleavages at 90 degrees and a hardness of about 6 on the Mohs scale, while Microcline displays a triclinic crystal system with a characteristic grid-like pattern of twinning called tartan twinning and is slightly harder with a similar Mohs hardness of 6 to 6.5. Color variations are common in Orthoclase, ranging from white to pink or yellow, whereas Microcline often appears in green shades, including amazonite, highlighting key differences relevant for mineral identification and industrial applications.
Occurrence and Geological Formation
Orthoclase and microcline are both feldspar minerals commonly found in igneous and metamorphic rocks, with orthoclase typically forming in granitic and syenitic environments due to slower cooling rates. Microcline, a polymorph of orthoclase, usually forms in similar granitic settings but often crystallizes at lower temperatures during the final stages of cooling or metamorphism, leading to its distinctive triclinic structure. These minerals frequently occur together in pegmatites and metamorphic terrains, where variations in temperature and pressure influence their stability and transformation.
Color and Appearance
Orthoclase typically exhibits a pearly luster with colors ranging from white, pink, and cream to light shades of green or yellow, often showing a more translucent appearance. Microcline varies slightly with a characteristic grid-like twinning pattern and usually displays colors from pale green to reddish or salmon pink, which can give it a more opaque appearance compared to Orthoclase. Both feldspar minerals share similar crystal structures but can be distinguished visually by Microcline's distinctive cross-hatched twinning and subtle color variations.
Industrial and Gemstone Uses
Orthoclase and microcline, both potassium feldspars, differ in crystal structure, impacting their industrial and gemstone applications. Orthoclase's higher clarity and stability make it preferred in gemstone cutting, especially for moonstones, while microcline's distinct grid-like twinning is prized in amazonite varieties. Industrially, orthoclase serves as a key ingredient in glassmaking and ceramics due to its high potassium content, whereas microcline is valued for its durability in tile and porcelain production.
Identification Techniques
Orthoclase and microcline, both potassium feldspars, can be differentiated through identification techniques such as polarized light microscopy, where microcline exhibits cross-hatched twinning patterns distinct from the simple twinning in orthoclase. X-ray diffraction (XRD) analysis reveals microcline's triclinic crystal structure compared to the monoclinic structure of orthoclase, providing precise mineral identification. Additionally, differential thermal analysis (DTA) can assist by highlighting differences in phase transition temperatures unique to each mineral.
Orthoclase vs Microcline: Key Distinguishing Features
Orthoclase and Microcline are both potassium feldspar minerals but differ primarily in their crystal structure and optical properties, with Orthoclase exhibiting a monoclinic crystal system while Microcline has a triclinic structure. Orthoclase often shows carlsbad twinning, whereas Microcline displays characteristic grid or tartan twinning under polarized light. These differences influence their stability and occurrence, with Microcline typically forming at lower temperatures and being more common in granitic rocks compared to Orthoclase.
Summary and Conclusion
Orthoclase and microcline, both potassium feldspars, exhibit distinct crystal structures, with microcline displaying a triclinic form and orthoclase a monoclinic form, influencing their physical properties and stability. The transformation from orthoclase to microcline occurs through slow cooling, resulting in microcline's characteristic grid-like tartan twinning. Understanding these differences is crucial in petrology for accurate mineral identification and interpreting geological histories.
Orthoclase and Microcline Infographic
 libterm.com
libterm.com