Polyclonal antibodies consist of a diverse mixture of antibody molecules that recognize multiple epitopes on a single antigen, enhancing the immune response's flexibility and strength. They are widely used in research, diagnostics, and therapeutic applications due to their ability to detect a broad range of antigenic sites. Discover how polyclonal antibodies can benefit your studies by exploring the detailed insights in this article.
Table of Comparison
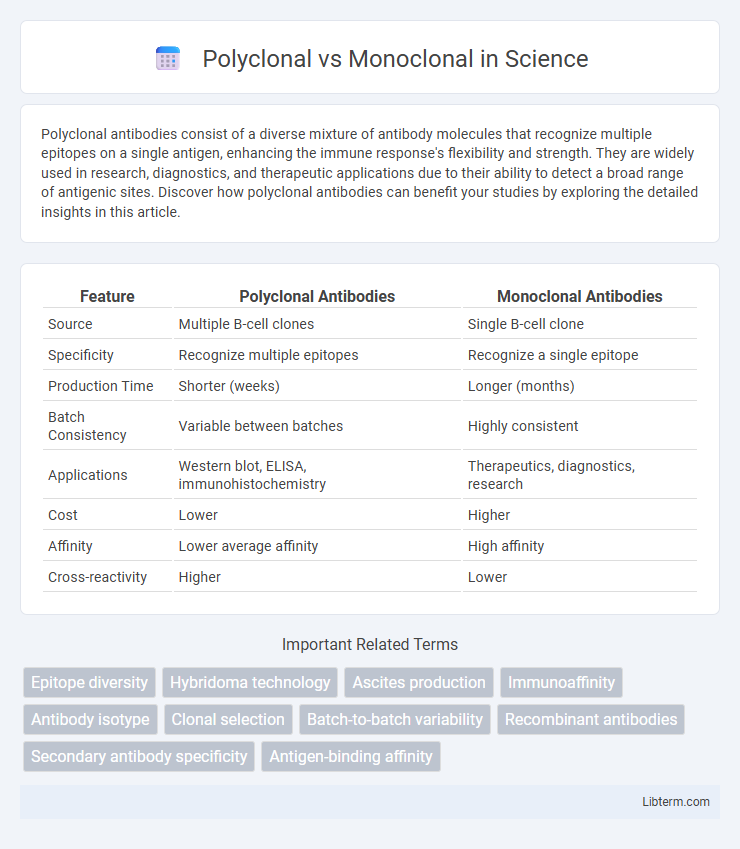
| Feature | Polyclonal Antibodies | Monoclonal Antibodies |
|---|---|---|
| Source | Multiple B-cell clones | Single B-cell clone |
| Specificity | Recognize multiple epitopes | Recognize a single epitope |
| Production Time | Shorter (weeks) | Longer (months) |
| Batch Consistency | Variable between batches | Highly consistent |
| Applications | Western blot, ELISA, immunohistochemistry | Therapeutics, diagnostics, research |
| Cost | Lower | Higher |
| Affinity | Lower average affinity | High affinity |
| Cross-reactivity | Higher | Lower |
Understanding Polyclonal and Monoclonal Antibodies
Polyclonal antibodies are a diverse mixture of immunoglobulins produced by different B cell clones, targeting multiple epitopes on a single antigen, which enhances detection sensitivity and robustness in assays. Monoclonal antibodies are homogenous immunoglobulins derived from a single B cell clone, ensuring specificity by binding to a single epitope with consistent affinity, ideal for therapeutic and diagnostic applications. Understanding the differences in production, specificity, and application is essential for selecting the appropriate antibody type in research and clinical diagnostics.
Origins and Production Methods
Polyclonal antibodies originate from multiple B-cell clones, produced by immunizing an animal and collecting serum containing diverse antibodies targeting various epitopes of an antigen. Monoclonal antibodies derive from a single B-cell clone, generated using hybridoma technology that fuses a specific antibody-producing B-cell with a myeloma cell to create a continuous, uniform antibody-producing line. The production of polyclonal antibodies involves bulk serum extraction, while monoclonal antibodies require cell culture or bioreactor systems for consistent, large-scale antibody generation.
Structural Differences Explained
Polyclonal antibodies consist of a diverse mixture of immunoglobulin molecules targeting multiple epitopes on the same antigen, produced by different B cell clones, resulting in heterogeneous structures. Monoclonal antibodies are identical immunoglobulins derived from a single B cell clone, targeting a specific epitope with uniform Fab regions and consistent molecular configuration. Structurally, monoclonal antibodies exhibit homogeneity in both variable and constant regions, while polyclonal antibodies display variability in antigen-binding sites due to the diverse B cell origins.
Specificity and Affinity Comparison
Polyclonal antibodies exhibit lower specificity and variable affinity due to their recognition of multiple epitopes on the same antigen, whereas monoclonal antibodies bind a single epitope with high specificity and consistent affinity. The affinity of monoclonal antibodies is typically higher and more uniform, making them ideal for precise diagnostic and therapeutic applications. Polyclonal antibodies' broad reactivity can be advantageous in detecting antigens with multiple epitopes but may result in cross-reactivity and reduced specificity.
Applications in Research and Diagnostics
Polyclonal antibodies, derived from multiple B-cell clones, offer broad reactivity and are widely utilized for detecting diverse epitopes in applications like Western blotting and immunohistochemistry, providing robust sensitivity in complex samples. Monoclonal antibodies, produced from a single B-cell clone, deliver high specificity and consistency, making them essential for precise diagnostic assays such as ELISA and flow cytometry, where targeted epitope recognition is critical. Both antibody types play pivotal roles in research and diagnostics, with polyclonal antibodies excelling in binding heterogenous antigens and monoclonal antibodies favored for reproducible, quantitative analysis.
Therapeutic Uses in Medicine
Polyclonal antibodies, derived from multiple B-cell clones, offer broad-spectrum recognition beneficial for neutralizing diverse antigens in therapies like immune deficiency treatment and infectious disease control. Monoclonal antibodies, produced from a single B-cell clone with high specificity, are extensively used in targeted cancer therapies, autoimmune disease management, and precision diagnostics. Therapeutic applications leverage the polyclonal antibodies' ability to target multiple epitopes for wide-ranging immune responses, while monoclonal antibodies provide precise targeting, reducing off-target effects and improving treatment efficacy.
Advantages and Limitations of Polyclonal Antibodies
Polyclonal antibodies offer the advantage of recognizing multiple epitopes on a single antigen, enhancing sensitivity and detecting a wider variety of antigen isoforms or variants. They are typically faster and cheaper to produce compared to monoclonal antibodies, making them suitable for applications requiring broad reactivity or when antigen availability is limited. However, polyclonal antibodies suffer from batch-to-batch variability and lower specificity, which can result in higher background noise and cross-reactivity in experimental assays.
Benefits and Drawbacks of Monoclonal Antibodies
Monoclonal antibodies offer high specificity, targeting a single epitope which reduces off-target effects and enhances therapeutic precision. Their production is consistent and scalable, ensuring batch-to-batch uniformity critical for clinical applications. However, monoclonal antibodies may have limited effectiveness against antigen variants and can be costly to develop and manufacture compared to polyclonal antibodies.
Cost and Scalability Considerations
Polyclonal antibodies are generally less expensive to produce due to simpler immunization processes and quicker generation times, but they lack batch-to-batch consistency, affecting scalability in large-scale applications. Monoclonal antibodies require more complex and costly hybridoma technology or recombinant expression systems, yet they offer high reproducibility and consistent quality, making them more suitable for scalable industrial production. Cost-effectiveness in antibody selection depends on balancing initial investment against long-term needs for uniformity and volume in pharmaceutical or diagnostic manufacturing.
Choosing the Right Antibody for Your Needs
Selecting the right antibody depends on your research objectives, as polyclonal antibodies recognize multiple epitopes on a single antigen, providing stronger signal and greater sensitivity, while monoclonal antibodies bind to a single epitope, offering higher specificity and reproducibility. Polyclonal antibodies are beneficial for detecting proteins in complex samples or when antigen variability exists, whereas monoclonal antibodies are favored for diagnostic assays and therapeutic applications due to batch-to-batch consistency. Understanding the advantages and limitations of each type ensures optimal detection, quantification, and validation in applications such as Western blotting, immunohistochemistry, or flow cytometry.
Polyclonal Infographic
 libterm.com
libterm.com