Catabolic processes break down complex molecules into simpler ones, releasing energy essential for cellular functions. This metabolic pathway plays a critical role in maintaining your body's energy balance and supporting overall health. Explore the rest of the article to understand how catabolism impacts your metabolism and well-being.
Table of Comparison
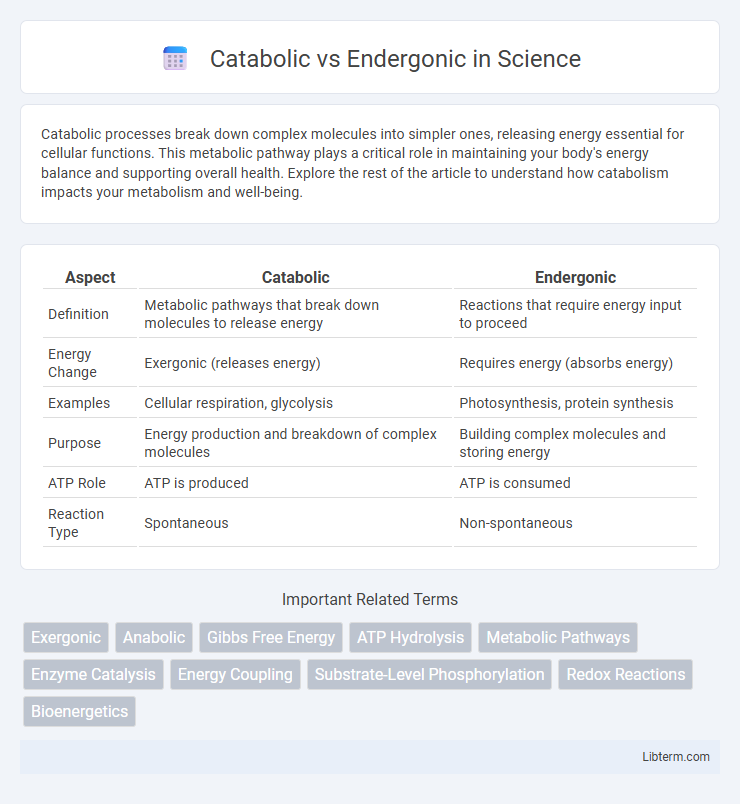
| Aspect | Catabolic | Endergonic |
|---|---|---|
| Definition | Metabolic pathways that break down molecules to release energy | Reactions that require energy input to proceed |
| Energy Change | Exergonic (releases energy) | Requires energy (absorbs energy) |
| Examples | Cellular respiration, glycolysis | Photosynthesis, protein synthesis |
| Purpose | Energy production and breakdown of complex molecules | Building complex molecules and storing energy |
| ATP Role | ATP is produced | ATP is consumed |
| Reaction Type | Spontaneous | Non-spontaneous |
Understanding Catabolic and Endergonic Processes
Catabolic processes involve the breakdown of complex molecules into simpler ones, releasing energy stored in chemical bonds. Endergonic processes require energy input to synthesize complex molecules from simpler precursors, storing energy in newly formed bonds. Understanding the energy flow and molecular transformations in catabolic and endergonic pathways is essential for studying cellular metabolism and bioenergetics.
Definition of Catabolic Reactions
Catabolic reactions involve the breakdown of complex molecules into simpler ones, releasing energy stored in chemical bonds. These exergonic processes contrast with endergonic reactions, which require an input of energy to synthesize complex molecules. In cellular metabolism, catabolic pathways provide the energy necessary for various biological functions by degrading carbohydrates, lipids, and proteins.
Definition of Endergonic Reactions
Endergonic reactions are chemical processes that require an input of energy to proceed, resulting in products that have higher free energy than the reactants. These reactions are non-spontaneous and play a crucial role in biological systems by driving essential processes such as photosynthesis and active transport. In contrast to catabolic reactions that release energy, endergonic reactions store energy within chemical bonds, making them vital for cellular growth and metabolism.
Key Differences Between Catabolic and Endergonic
Catabolic processes involve the breakdown of complex molecules into simpler ones, releasing energy that cells can harness for biological functions. Endergonic reactions require an input of energy to proceed, as they involve the synthesis of complex molecules from simpler precursors. Key differences include that catabolic pathways are typically exergonic, releasing free energy, while endergonic pathways consume energy, often coupling with ATP hydrolysis to drive non-spontaneous reactions.
Biological Significance of Catabolic Reactions
Catabolic reactions play a crucial role in cellular metabolism by breaking down complex molecules like carbohydrates, lipids, and proteins into simpler units, releasing energy stored in chemical bonds. This released energy is often captured in the form of adenosine triphosphate (ATP), which fuels various endergonic processes essential for cell growth, repair, and maintenance. Efficient catabolic pathways such as glycolysis, the citric acid cycle, and oxidative phosphorylation are vital for sustaining life by providing the energy currency necessary for anabolic reactions and overall cellular function.
Biological Role of Endergonic Reactions
Endergonic reactions play a crucial biological role by driving energy-requiring processes such as biosynthesis, active transport, and cell signaling, enabling organisms to build complex molecules and maintain cellular functions. These reactions absorb free energy from their surroundings, often coupling with exergonic reactions like ATP hydrolysis to proceed efficiently. In contrast to catabolic pathways that break down molecules to release energy, endergonic pathways are essential for growth, repair, and maintaining metabolic balance.
Examples of Catabolic Pathways
Catabolic pathways break down complex molecules into simpler ones, releasing energy essential for cellular activities. Examples include glycolysis, which converts glucose into pyruvate while generating ATP, and the citric acid cycle, where acetyl-CoA is oxidized to CO2, producing NADH and FADH2 for ATP synthesis. Another key catabolic pathway is beta-oxidation, which degrades fatty acids into acetyl-CoA units, fueling energy production in mitochondria.
Examples of Endergonic Pathways
Photosynthesis exemplifies an endergonic pathway where energy from sunlight drives the synthesis of glucose from carbon dioxide and water, requiring a net input of energy. Another example is the synthesis of proteins from amino acids, which consumes ATP to form peptide bonds during translation. The Calvin cycle in plants also represents an endergonic process, utilizing ATP and NADPH to fix carbon and produce sugars.
Energy Transformation in Catabolic vs Endergonic
Catabolic processes release energy by breaking down complex molecules into simpler ones, generating ATP and heat essential for cellular activities. Endergonic reactions require energy input, often sourced from ATP hydrolysis, to synthesize complex molecules from simpler precursors. The energy transformation in catabolic pathways fuels endergonic processes, maintaining cellular metabolism and homeostasis.
Catabolic and Endergonic: Importance in Metabolism
Catabolic processes break down complex molecules into simpler ones, releasing energy essential for cellular functions and maintaining metabolic balance. Endergonic reactions require an input of energy to synthesize complex molecules, driving biosynthetic pathways critical for cell growth and repair. The interplay between catabolic energy release and endergonic energy consumption underpins metabolic regulation and energy homeostasis in living organisms.
Catabolic Infographic
 libterm.com
libterm.com