Ferrimagnetic materials exhibit spontaneous magnetization due to the unequal antiparallel alignment of magnetic moments in their structure, resulting in a net magnetic moment. These materials are crucial in applications such as magnetic storage, transformers, and spintronics due to their unique magnetic properties. Explore the rest of this article to deepen your understanding of ferrimagnetic behavior and its technological significance.
Table of Comparison
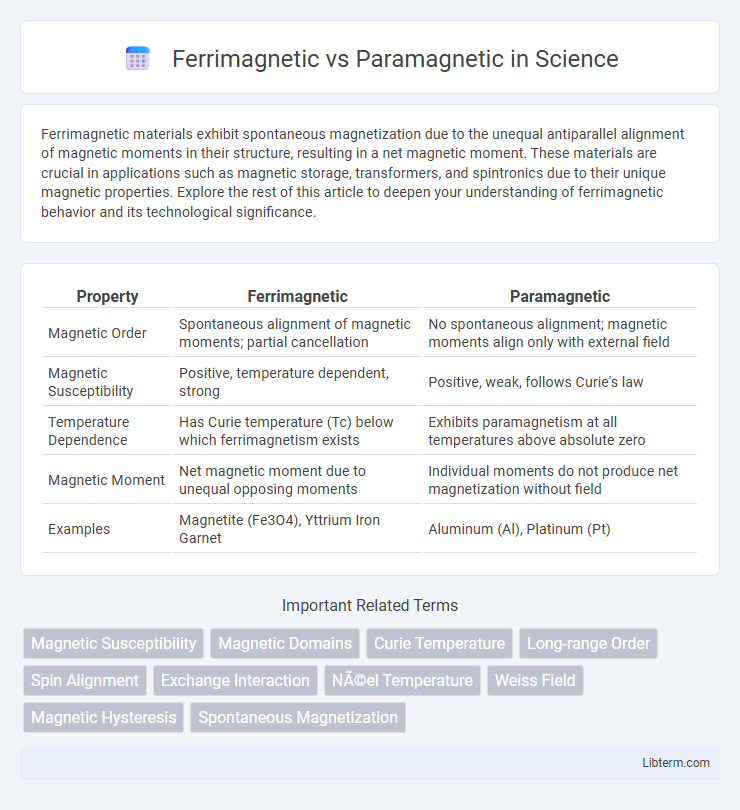
| Property | Ferrimagnetic | Paramagnetic |
|---|---|---|
| Magnetic Order | Spontaneous alignment of magnetic moments; partial cancellation | No spontaneous alignment; magnetic moments align only with external field |
| Magnetic Susceptibility | Positive, temperature dependent, strong | Positive, weak, follows Curie's law |
| Temperature Dependence | Has Curie temperature (Tc) below which ferrimagnetism exists | Exhibits paramagnetism at all temperatures above absolute zero |
| Magnetic Moment | Net magnetic moment due to unequal opposing moments | Individual moments do not produce net magnetization without field |
| Examples | Magnetite (Fe3O4), Yttrium Iron Garnet | Aluminum (Al), Platinum (Pt) |
Introduction to Magnetic Materials
Ferrimagnetic materials exhibit spontaneous magnetization due to the unequal opposing magnetic moments of atoms, resulting in a net magnetic moment even without an external field. Paramagnetic materials contain unpaired electrons that align with an external magnetic field but lack intrinsic magnetization, causing a weak and temporary magnetic response. Understanding these differences is crucial for applications in magnetic storage, sensors, and electronic devices, where material-specific magnetic behavior defines performance.
What is Ferrimagnetism?
Ferrimagnetism is a type of magnetic ordering where magnetic moments of atoms on different sublattices are opposed but unequal in magnitude, resulting in a net spontaneous magnetization similar to ferromagnetism. This phenomenon is commonly observed in ferrites, materials composed of iron oxides combined with other metal elements, which exhibit strong magnetic properties used in memory devices and transformers. Unlike paramagnetism, where magnetization occurs only in the presence of an external magnetic field and is generally weak, ferrimagnetic materials retain magnetization even after the external field is removed due to their intrinsic atomic structure.
What is Paramagnetism?
Paramagnetism is a form of magnetism characterized by the presence of unpaired electrons in atomic or molecular structures, causing materials to be weakly attracted to external magnetic fields. Unlike ferrimagnetic materials, which exhibit spontaneous magnetization due to unequal opposing magnetic moments, paramagnetic substances only align with magnetic fields temporarily and do not retain magnetization once the field is removed. This magnetic behavior is temperature-dependent and is quantitatively described by Curie's law, where magnetic susceptibility inversely correlates with temperature.
Atomic Structure Differences
Ferrimagnetic materials contain atoms with magnetic moments aligned in opposite directions but with unequal magnitudes, resulting in a net magnetic moment due to incomplete cancellation at the atomic level. Paramagnetic materials consist of atoms or ions with unpaired electrons that have magnetic moments randomly oriented, leading to no net magnetization without an external magnetic field. The key atomic structural difference lies in the arrangement and interaction of atomic magnetic moments, where ferrimagnetism arises from antiparallel ordering with unequal moments, contrasting with the isolated and thermally agitated moments in paramagnetism.
Magnetic Moment Alignment
Ferrimagnetic materials exhibit magnetic moments aligned in opposite directions, but with unequal magnitudes, resulting in a net magnetic moment and spontaneous magnetization. Paramagnetic materials have randomly oriented magnetic moments that align weakly and temporarily with an external magnetic field, producing no intrinsic magnetization. The difference in magnetic moment alignment governs the strong, permanent magnetism in ferrimagnets versus the weak, induced magnetism in paramagnets.
Temperature Influence on Magnetism
Ferrimagnetic materials exhibit a strong temperature dependence in magnetism, with their magnetic order weakening and eventually disappearing above the Curie temperature due to thermal agitation disrupting the alignment of opposing magnetic moments. Paramagnetic substances show increased magnetization with decreasing temperature, as thermal energy reduces the random orientation of magnetic moments, enhancing their alignment with an external magnetic field. The interplay between thermal energy and magnetic ordering distinctly influences the magnetic behavior in ferrimagnetic and paramagnetic materials.
Real-World Examples of Ferrimagnetism
Ferrimagnetism is prominently exhibited in materials such as magnetite (Fe3O4) and ferrites used in magnetic recording media and transformers, where unequal opposing magnetic moments result in spontaneous magnetization. Unlike paramagnetic materials like aluminum or platinum, which only exhibit magnetism in the presence of an external magnetic field, ferrimagnetic substances maintain magnetic ordering due to electron spin alignment. These real-world examples highlight ferrimagnetism's critical role in data storage technologies and electromagnetic devices.
Common Paramagnetic Materials
Common paramagnetic materials include aluminum, platinum, and tungsten, which exhibit weak and positive magnetic susceptibility due to unpaired electrons aligning with an external magnetic field. In contrast, ferrimagnetic materials like magnetite show strong magnetic ordering with unequal opposing magnetic moments resulting in a net magnetization. Paramagnetic behavior is typically temperature-dependent and weak compared to the spontaneous magnetization characteristic of ferrimagnetism.
Applications in Technology
Ferrimagnetic materials are widely used in magnetic storage devices such as hard disk drives and magnetic sensors due to their strong spontaneous magnetization and stable magnetic domains. Paramagnetic materials find applications in contrast agents for magnetic resonance imaging (MRI) because of their weak and temporary magnetization in the presence of an external magnetic field. The distinct magnetic behaviors of ferrimagnetic and paramagnetic substances enable their tailored use in data storage, medical imaging, and spintronic devices.
Key Differences: Ferrimagnetic vs Paramagnetic
Ferrimagnetic materials exhibit spontaneous magnetization due to antiparallel but unequal magnetic moments, resulting in a net magnetic moment, whereas paramagnetic materials only show magnetization in the presence of an external magnetic field, with magnetic moments aligned randomly at rest. Ferrimagnetism occurs in compounds like magnetite (Fe3O4), while paramagnetism is observed in materials such as aluminum and oxygen. The temperature dependence differs significantly: ferrimagnetic materials have a Curie temperature below which they retain magnetization, while paramagnetic materials follow Curie's law and lose magnetization without an external field.
Ferrimagnetic Infographic
 libterm.com
libterm.com