Supersaturated solutions contain more dissolved solute than what is typically possible at equilibrium under normal conditions, resulting in a metastable state prone to crystallization. This phenomenon is crucial in various applications, from chemical manufacturing to pharmaceuticals, where controlling solubility impacts product quality and efficiency. Discover how understanding supersaturation can enhance your control over these processes by exploring the detailed insights in the rest of this article.
Table of Comparison
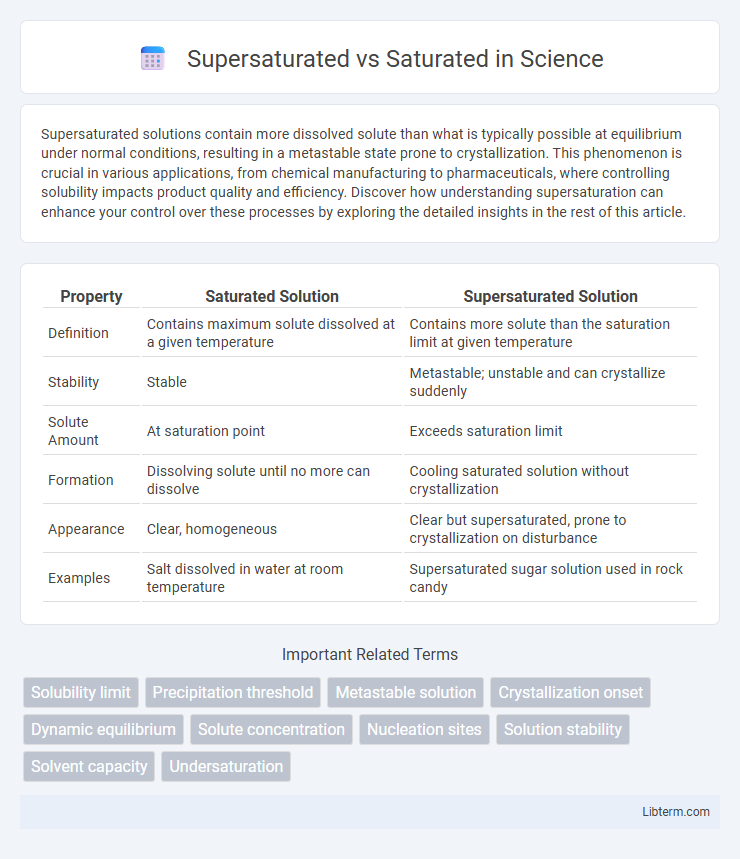
| Property | Saturated Solution | Supersaturated Solution |
|---|---|---|
| Definition | Contains maximum solute dissolved at a given temperature | Contains more solute than the saturation limit at given temperature |
| Stability | Stable | Metastable; unstable and can crystallize suddenly |
| Solute Amount | At saturation point | Exceeds saturation limit |
| Formation | Dissolving solute until no more can dissolve | Cooling saturated solution without crystallization |
| Appearance | Clear, homogeneous | Clear but supersaturated, prone to crystallization on disturbance |
| Examples | Salt dissolved in water at room temperature | Supersaturated sugar solution used in rock candy |
Understanding Saturated Solutions
Saturated solutions contain the maximum amount of solute that can dissolve at a specific temperature and pressure, beyond which no more solute can dissolve. This equilibrium state is crucial for predicting solubility limits in chemical processes and environmental conditions. Understanding saturated solutions helps in applications like crystallization, pharmaceutical formulation, and industrial solvent recovery.
What is a Supersaturated Solution?
A supersaturated solution contains more dissolved solute than a saturated solution at the same temperature, achieved by carefully cooling a saturated solution without crystallization. This metastable state holds excess solute temporarily, making it highly sensitive to disturbances that trigger rapid crystallization. Supersaturated solutions are important in fields like chemistry and pharmaceuticals for controlled crystallization and solubility studies.
Key Differences: Saturated vs Supersaturated
Saturated solutions contain the maximum amount of solute dissolved at a given temperature and pressure, while supersaturated solutions hold more solute than the equilibrium limit, achieved by carefully cooling or evaporating the solvent without crystallization. Supersaturated solutions are unstable and prone to rapid crystallization upon disturbance, contrary to the stable equilibrium state of saturated solutions. This distinction is critical in industries like pharmaceuticals and crystallography, where precise control over solubility influences product formulation and crystal growth.
Formation of Saturated Solutions
Saturated solutions form when a solute dissolves in a solvent until no more solute can be dissolved at a given temperature and pressure, reaching equilibrium. Supersaturated solutions occur when the solution contains more dissolved solute than the saturation point, usually created by dissolving solute at a higher temperature and then carefully cooling the solution. The formation of saturated solutions depends on factors such as temperature, solvent properties, and solute solubility limits.
How Supersaturation Occurs
Supersaturation occurs when a solution contains more dissolved solute than it can theoretically hold at a given temperature, typically achieved by heating the solvent to dissolve excess solute and then slowly cooling it without disturbing the solution. This metastable state arises because the solute remains dissolved beyond its equilibrium concentration, as no nucleation sites are present to trigger crystallization. Factors such as cooling rate, purity of the solution, and absence of seed crystals influence the formation and stability of a supersaturated solution compared to a saturated one.
Visual Indicators: Identifying Each Type
Supersaturated solutions often exhibit crystal formation or precipitation upon slight disturbance, indicating excess solute beyond saturation limits. Saturated solutions remain clear and stable, showing no visible changes as they contain the maximum dissolved solute at a given temperature. Observing turbidity or solid deposits provides a key visual indicator to distinguish supersaturated from saturated solutions.
Practical Applications in Industry
Supersaturated solutions are critical in industries such as pharmaceuticals and crystallization processes where precise control of solute concentration enables the formation of high-purity crystals or targeted drug delivery systems. Saturated solutions find extensive use in chemical manufacturing and food processing, providing consistent solubility boundaries essential for controlled reactions and product stability. Understanding the differences between supersaturated and saturated states allows engineers to optimize processes like precipitation, crystallization, and solubility-based separations for enhanced efficiency and product quality.
Real-World Examples
Supersaturated solutions occur when a solution contains more solute than its equilibrium saturation point, such as in the case of sugar dissolved in hot water that remains dissolved upon cooling, leading to crystal formation when disturbed. Saturated solutions are regularly encountered in everyday life, like saltwater at equilibrium where no more salt can dissolve at a given temperature. The difference is critical in industrial processes like pharmaceuticals, where supersaturation enables controlled crystallization of drugs to enhance solubility and bioavailability.
Benefits and Risks of Supersaturated Solutions
Supersaturated solutions offer enhanced solute concentration beyond saturation, enabling increased chemical reaction rates and improved crystallization processes essential in industries such as pharmaceuticals and materials science. However, these solutions pose risks of sudden crystallization or precipitation, which can lead to process instability or product inconsistencies. Proper control of temperature and solute concentration is crucial to harness the benefits while minimizing the hazards associated with supersaturation.
Frequently Asked Questions
Supersaturated solutions contain more dissolved solute than a saturated solution at the same temperature, leading to instability and potential crystallization upon disturbance. Saturated solutions hold the maximum amount of solute that can dissolve under specific conditions, maintaining equilibrium between dissolved and undissolved solute. Common questions address how supersaturation occurs, how to identify supersaturated solutions, and their applications in industries like crystallization and pharmaceuticals.
Supersaturated Infographic
 libterm.com
libterm.com