A cofactor is a non-protein chemical compound essential for the biological activity of certain enzymes, often facilitating the enzyme's catalytic function. These molecules can be metal ions or organic compounds known as coenzymes, playing a crucial role in metabolic processes. Explore the rest of the article to understand how cofactors influence your body's biochemical reactions.
Table of Comparison
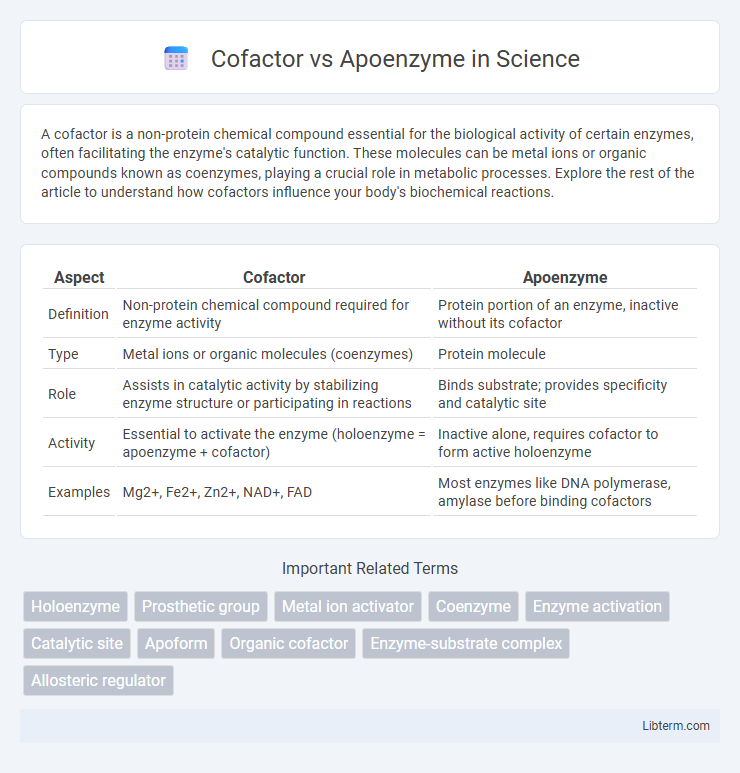
| Aspect | Cofactor | Apoenzyme |
|---|---|---|
| Definition | Non-protein chemical compound required for enzyme activity | Protein portion of an enzyme, inactive without its cofactor |
| Type | Metal ions or organic molecules (coenzymes) | Protein molecule |
| Role | Assists in catalytic activity by stabilizing enzyme structure or participating in reactions | Binds substrate; provides specificity and catalytic site |
| Activity | Essential to activate the enzyme (holoenzyme = apoenzyme + cofactor) | Inactive alone, requires cofactor to form active holoenzyme |
| Examples | Mg2+, Fe2+, Zn2+, NAD+, FAD | Most enzymes like DNA polymerase, amylase before binding cofactors |
Introduction to Enzymes and Their Components
Enzymes are biological catalysts composed of protein structures that accelerate metabolic reactions by lowering activation energy. Apoenzymes are the inactive protein portions of enzymes that require non-protein molecules called cofactors to become catalytically active. Cofactors can be metal ions, such as magnesium or zinc, or organic molecules known as coenzymes, both essential for the enzyme's proper functioning and substrate binding.
Defining Cofactors and Apoenzymes
Cofactors are non-protein chemical compounds or metallic ions essential for the biological activity of certain enzymes, often classified as either organic molecules called coenzymes or inorganic ions such as metal ions like Mg2+ or Fe2+. Apoenzymes represent the protein component of an enzyme without its cofactor, and they are catalytically inactive until the cofactor binds, forming the active holoenzyme complex. The interaction between apoenzymes and cofactors is crucial for enzyme specificity and catalytic efficiency in metabolic reactions.
Types of Cofactors: Organic vs Inorganic
Cofactors are non-protein chemical compounds essential for enzyme activity, classified into organic and inorganic types. Organic cofactors, known as coenzymes, include vitamins like niacin and riboflavin that facilitate enzyme reactions by transferring functional groups. Inorganic cofactors typically consist of metal ions such as Mg2+, Fe2+, and Zn2+, which stabilize enzyme structure or participate directly in catalytic processes.
Apoenzyme: Structure and Function
Apoenzymes are the protein portions of enzymes that require cofactors to become catalytically active, characterized by their specific tertiary structure which provides the active site for substrate binding. They are inactive alone, relying on the binding of metal ions or organic molecules (cofactors) to form a holoenzyme, which stabilizes the enzyme-substrate complex and facilitates biochemical reactions. The structure of apoenzymes ensures specificity and regulation in metabolic pathways by determining the precise interaction with its cofactor and substrate.
Cofactor Binding: Mechanism and Importance
Cofactor binding involves the attachment of non-protein molecules or ions to an apoenzyme, converting it into an active holoenzyme essential for catalytic activity. This interaction typically occurs through specific non-covalent forces such as hydrogen bonds, ionic interactions, and van der Waals forces, ensuring precise positioning of the cofactor within the enzyme's active site. Proper cofactor binding stabilizes the enzyme-substrate complex, enhances catalytic efficiency, and is critical for the biochemical processes that depend on enzyme functionality.
Holoenzyme Formation: Apoenzyme plus Cofactor
Holoenzyme formation occurs when an apoenzyme, the inactive protein portion of an enzyme, binds to a cofactor, which can be a metal ion or an organic molecule essential for enzymatic activity. This binding induces a conformational change that activates the holoenzyme, enabling it to catalyze specific biochemical reactions efficiently. The interaction between apoenzyme and cofactor is critical for the functionality of many enzymes involved in metabolic pathways.
Key Differences: Cofactor vs Apoenzyme
Cofactors are non-protein chemical compounds, such as metal ions or organic molecules, that assist enzymes in catalyzing reactions by stabilizing enzyme structure or participating directly in the reaction. Apoenzymes refer to the inactive form of enzymes, consisting solely of the protein component without their required cofactor or coenzyme, thus unable to perform catalytic activity. The key difference lies in that cofactors are essential activators or helpers for enzymatic function, whereas apoenzymes represent the incomplete, non-functional enzyme lacking these necessary cofactors.
Biological Roles in Metabolic Pathways
Cofactors, which can be metal ions or organic molecules, are essential for activating apoenzymes by enabling their catalytic activity in metabolic pathways. Apoenzymes, the inactive protein components of enzymes, require specific cofactors to form holoenzymes that facilitate biochemical reactions such as substrate binding and product formation. In metabolic pathways, the presence of appropriate cofactors ensures proper enzyme function, regulating critical processes like energy production, biosynthesis, and detoxification.
Clinical Implications and Deficiencies
Cofactors, including metal ions and organic molecules, are essential for the activation of apoenzymes into functional holoenzymes, impacting enzymatic reactions critical for metabolic pathways. Deficiencies in cofactors like vitamin-derived coenzymes (e.g., NAD+, FAD) or metal ions (e.g., zinc, magnesium) can lead to clinical disorders such as beriberi, pellagra, or anemia due to impaired enzymatic activity. Understanding the clinical implications of cofactor deficiencies aids in diagnosing metabolic diseases and informs targeted vitamin or mineral supplementation therapies.
Summary Table: Cofactor vs Apoenzyme
Cofactors are non-protein chemical compounds, such as metal ions or organic molecules, essential for enzyme activity, whereas apoenzymes are the protein components of enzymes lacking their necessary cofactors. Binding of a cofactor to an apoenzyme forms a holoenzyme, which is catalytically active. Key differences highlighted in the summary table include the cofactor's role as an activator and the apoenzyme's dependence on cofactor presence for functional enzymatic activity.
Cofactor Infographic
 libterm.com
libterm.com