Non-immunogenic substances do not provoke an immune response when introduced into the body, making them ideal for various medical applications such as drug delivery and implantable devices. Understanding how non-immunogenic materials interact with the immune system helps improve biocompatibility and reduces adverse reactions. Explore the rest of the article to learn how non-immunogenicity enhances therapeutic safety and effectiveness.
Table of Comparison
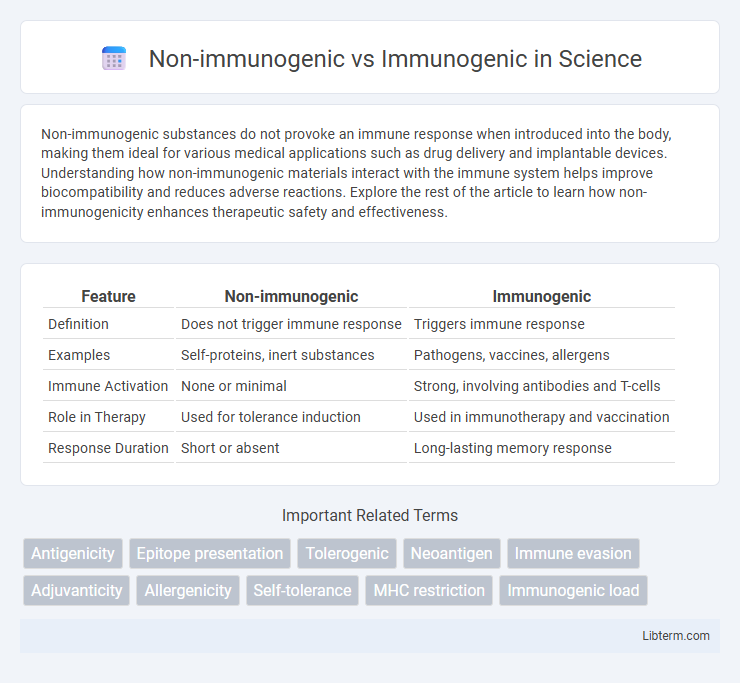
| Feature | Non-immunogenic | Immunogenic |
|---|---|---|
| Definition | Does not trigger immune response | Triggers immune response |
| Examples | Self-proteins, inert substances | Pathogens, vaccines, allergens |
| Immune Activation | None or minimal | Strong, involving antibodies and T-cells |
| Role in Therapy | Used for tolerance induction | Used in immunotherapy and vaccination |
| Response Duration | Short or absent | Long-lasting memory response |
Understanding Immunogenicity: Key Concepts
Immunogenicity refers to the ability of a substance, such as an antigen or therapeutic protein, to provoke an immune response in the body. Non-immunogenic compounds do not trigger immune activation, making them preferable in drug design to avoid adverse immune reactions. Understanding factors influencing immunogenicity, including molecular structure, dosage, and patient-specific variables, is critical for developing safe and effective immunotherapies.
Definition of Non-immunogenic Reactions
Non-immunogenic reactions refer to biological responses that do not involve the activation of the immune system or the production of antibodies. These reactions typically arise from direct toxic effects, pharmacological actions, or physical irritation without triggering immune memory or hypersensitivity. Understanding the distinction between non-immunogenic and immunogenic responses is crucial in drug development to minimize adverse immune-mediated effects and improve therapeutic safety.
What Makes a Substance Immunogenic?
A substance's immunogenicity depends on its molecular size, complexity, and ability to be recognized by the immune system as foreign, which triggers an immune response. Protein-based antigens are typically immunogenic due to their complex structures and presence of epitopes that activate T cells and B cells. Small molecules, or haptens, often become immunogenic only when attached to larger carrier proteins, highlighting the importance of antigen processing and presentation in immune recognition.
Biological Mechanisms Behind Immunogenicity
Non-immunogenic substances typically evade immune recognition due to their molecular structure lacking antigenic determinants or being similar to self-antigens, preventing activation of T and B cells. Immunogenic molecules contain epitopes that are processed by antigen-presenting cells, leading to MHC presentation and subsequent activation of adaptive immunity. The strength of immune response depends on factors such as antigen size, complexity, and the presence of costimulatory signals that modulate immunogenicity at the cellular and molecular levels.
Factors Influencing Non-immunogenic Properties
Non-immunogenic properties are influenced by factors such as molecular size, structural similarity to host proteins, and dosage frequency, which minimize immune system activation. Modification techniques like PEGylation and glycosylation increase biological compatibility and reduce antigenicity. Understanding these factors is crucial for developing therapeutic proteins with low immunogenic risk and improved clinical safety.
Clinical Implications of Immunogenic vs Non-immunogenic Agents
Immunogenic agents trigger an immune response that can enhance therapeutic efficacy or cause adverse effects, such as hypersensitivity or reduced drug effectiveness due to antibody formation. Non-immunogenic agents minimize immune activation, resulting in improved safety profiles and consistent patient outcomes with lower risk of immune-related complications. Understanding the immunogenicity of therapeutic agents is crucial for personalized medicine, vaccine development, and optimizing treatment regimens in clinical practice.
Applications in Drug Development
Non-immunogenic drug candidates minimize adverse immune responses, enhancing safety and efficacy in therapies, especially for chronic administration and biologics like monoclonal antibodies and enzyme replacement therapies. Immunogenic compounds stimulate immune activation, useful in vaccine development and cancer immunotherapies to provoke targeted immune responses against pathogens or tumors. Balancing immunogenicity is critical in drug development to optimize therapeutic benefits while reducing risks of hypersensitivity and immune clearance.
Immunogenicity in Vaccine Design
Immunogenicity in vaccine design determines the ability of a vaccine to provoke a robust immune response, critical for effective protection against pathogens. Immunogenic vaccines contain antigens that activate B cells and T cells, leading to memory cell formation for long-lasting immunity. Conversely, non-immunogenic components fail to elicit significant immune activation, weakening vaccine efficacy and necessitating the use of adjuvants to enhance immune recognition.
Strategies to Reduce Immunogenicity
Non-immunogenic materials minimize immune system activation by avoiding recognition through molecular mimicry and reduced antigen presentation. Strategies to reduce immunogenicity include surface modification with polyethylene glycol (PEGylation), use of biomimetic coatings, and incorporating immunosuppressive agents to evade or suppress immune responses. Engineering proteins to remove epitopes and employing tolerance induction techniques further decrease immunogenic potential in therapeutic applications.
Future Perspectives in Immunogenicity Research
Future perspectives in immunogenicity research emphasize the development of advanced predictive models to distinguish non-immunogenic from immunogenic compounds, enhancing therapeutic safety and efficacy. Integration of machine learning algorithms with high-throughput sequencing and proteomics data will enable more accurate identification of immune response triggers. Emerging biomaterials and nanotechnology are also being explored to design drug delivery systems that minimize immunogenicity while maximizing immune tolerance.
Non-immunogenic Infographic
 libterm.com
libterm.com