Mixed signals can create confusion and misunderstandings in communication, impacting both personal and professional relationships. Recognizing and interpreting these signals accurately is essential for clearer interactions and stronger connections. Explore the rest of the article to learn how you can better identify and respond to mixed signals effectively.
Table of Comparison
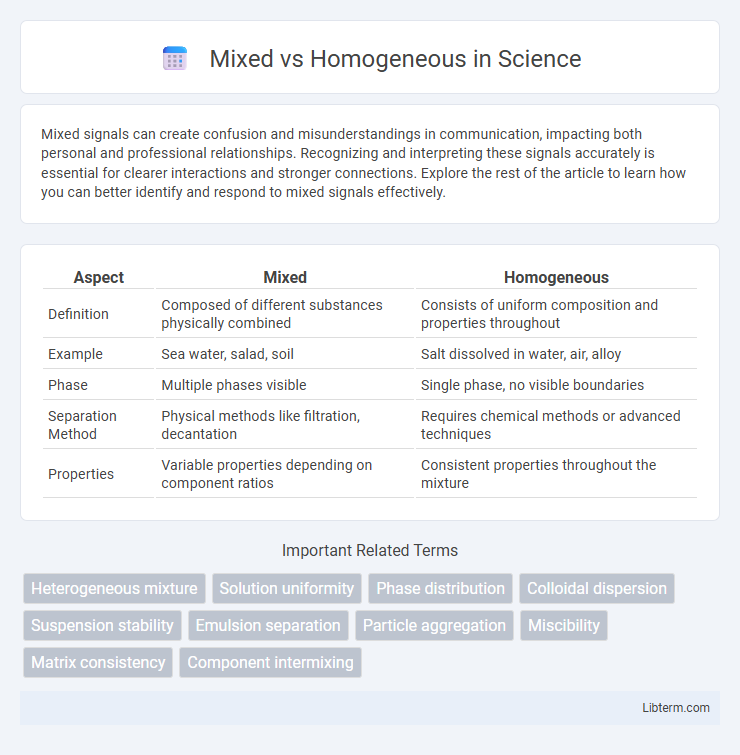
| Aspect | Mixed | Homogeneous |
|---|---|---|
| Definition | Composed of different substances physically combined | Consists of uniform composition and properties throughout |
| Example | Sea water, salad, soil | Salt dissolved in water, air, alloy |
| Phase | Multiple phases visible | Single phase, no visible boundaries |
| Separation Method | Physical methods like filtration, decantation | Requires chemical methods or advanced techniques |
| Properties | Variable properties depending on component ratios | Consistent properties throughout the mixture |
Introduction to Mixtures: Mixed vs Homogeneous
Mixtures can be classified as either homogeneous or heterogeneous based on their uniformity in composition. Homogeneous mixtures exhibit a consistent appearance and composition throughout, such as salt dissolved in water, while heterogeneous mixtures display distinct phases or components, like a salad or granite. Understanding these differences is essential for applications in chemistry, material science, and industrial processes where mixture properties impact functionality and performance.
Defining Homogeneous Mixtures
Homogeneous mixtures consist of two or more substances uniformly distributed at the molecular level, resulting in a single-phase composition. Examples include salt dissolved in water or air, where individual components are indistinguishable. These mixtures differ from heterogeneous or mixed mixtures, which have visibly distinct phases and non-uniform composition.
Understanding Heterogeneous Mixtures
Heterogeneous mixtures consist of two or more substances with visibly different phases or compositions, allowing easy identification of individual components, unlike homogeneous mixtures which have a uniform appearance throughout. Examples of heterogeneous mixtures include salad, soil, and granite, where solids, liquids, or gases remain distinct and are not evenly distributed. Understanding the physical separation methods such as filtration, decantation, and centrifugation is key to analyzing and separating components within heterogeneous mixtures.
Key Differences Between Mixed and Homogeneous Substances
Mixed substances consist of two or more components physically combined, retaining individual properties, while homogeneous substances have uniform composition and appearance throughout. Mixtures can be separated by physical means such as filtration or distillation, unlike homogeneous substances that are chemically bonded and require chemical processes for separation. Homogeneous substances include solutions and alloys, whereas mixed substances encompass mixtures like suspensions and colloids with visibly distinct phases.
Examples of Homogeneous Mixtures in Everyday Life
Salt dissolved in water, sugar dissolved in tea, and air are common examples of homogeneous mixtures found in everyday life. These mixtures have a uniform composition throughout, making the individual components indistinguishable. Other examples include vinegar, brass (an alloy of copper and zinc), and stainless steel, all of which maintain consistent properties throughout the sample.
Examples of Heterogeneous Mixtures Around Us
Heterogeneous mixtures consist of physically distinct components that are not uniformly distributed, such as salad dressing, where oil and vinegar separate visibly. Other common examples include soil, containing organic matter, minerals, and sand, and concrete, composed of cement, sand, gravel, and water. These mixtures demonstrate varied physical properties across their different regions, unlike homogeneous mixtures with consistent composition throughout.
Physical Properties: Appearance and Consistency
Mixed substances exhibit varied physical properties in appearance and consistency due to their heterogeneous composition, often displaying multiple colors, textures, or phases. Homogeneous substances maintain uniform appearance and consistent texture throughout, with a single phase that is visually indistinguishable in composition. Physical properties such as color, texture, and phase uniformity serve as key indicators to differentiate mixed from homogeneous materials.
Methods for Separating Mixed and Homogeneous Mixtures
Methods for separating mixed mixtures include filtration, decantation, and centrifugation, which exploit differences in particle size, density, and solubility. Homogeneous mixtures require techniques like distillation, chromatography, and crystallization that separate components based on boiling points, affinity to solvents, or solubility variations. Effective separation relies on understanding the physical and chemical properties specific to heterogeneous or homogeneous compositions.
Importance of Mixture Types in Science and Industry
Mixture types, specifically mixed and homogeneous mixtures, play a crucial role in scientific research and industrial applications due to their distinct physical and chemical properties. Homogeneous mixtures, with uniform composition, are essential in pharmaceuticals and chemical manufacturing for consistency and predictable reactions, while mixed or heterogeneous mixtures are pivotal in materials science and environmental engineering to study component interactions and optimize performance. Understanding the differences between these mixture types enables precise control over product quality, reaction efficiency, and process scalability in various scientific and industrial contexts.
Conclusion: Choosing the Right Mixture Type
Selecting the appropriate mixture type hinges on the specific application requirements, where homogeneous mixtures offer uniform composition and predictability, ideal for processes demanding consistency. Mixed or heterogeneous mixtures provide variable properties beneficial in applications requiring diverse functionalities or tailored performance. Evaluating factors such as desired uniformity, reactivity, and end-use conditions ensures optimal mixture selection for enhanced efficiency and effectiveness.
Mixed Infographic
 libterm.com
libterm.com