Anabolic processes are essential for building muscle, repairing tissues, and supporting overall growth by promoting the synthesis of complex molecules from simpler ones. Understanding how anabolic hormones like testosterone and insulin work can help optimize your fitness and health strategies. Explore the rest of the article to learn how to enhance your anabolic potential effectively.
Table of Comparison
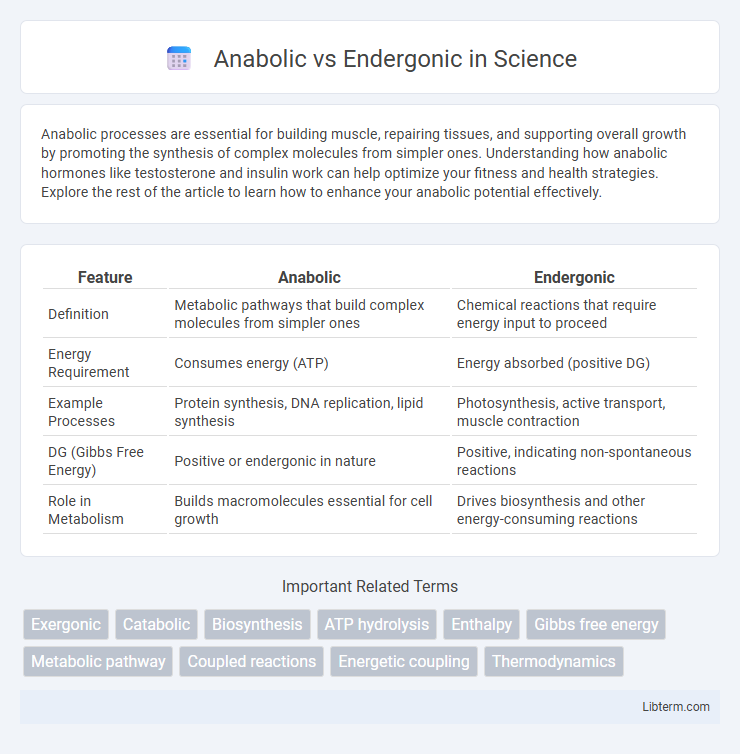
| Feature | Anabolic | Endergonic |
|---|---|---|
| Definition | Metabolic pathways that build complex molecules from simpler ones | Chemical reactions that require energy input to proceed |
| Energy Requirement | Consumes energy (ATP) | Energy absorbed (positive DG) |
| Example Processes | Protein synthesis, DNA replication, lipid synthesis | Photosynthesis, active transport, muscle contraction |
| DG (Gibbs Free Energy) | Positive or endergonic in nature | Positive, indicating non-spontaneous reactions |
| Role in Metabolism | Builds macromolecules essential for cell growth | Drives biosynthesis and other energy-consuming reactions |
Introduction to Anabolic and Endergonic Reactions
Anabolic reactions are biosynthetic processes that build complex molecules from simpler ones, requiring energy input to form chemical bonds. Endergonic reactions absorb energy from their surroundings, resulting in a positive Gibbs free energy change, indicating non-spontaneity without added energy. Both anabolic and endergonic reactions are crucial in cellular metabolism, with anabolic pathways typically being endergonic to support growth and biosynthesis.
Defining Anabolic Processes
Anabolic processes involve the synthesis of complex molecules from simpler ones, requiring energy input to form chemical bonds. These reactions are essential for cell growth, repair, and the storage of energy in macromolecules such as proteins, nucleic acids, and lipids. Anabolic pathways are tightly regulated and often couple with endergonic reactions to drive substrate assembly under physiological conditions.
Understanding Endergonic Reactions
Endergonic reactions require an input of energy to proceed and have a positive Gibbs free energy change (DG > 0), distinguishing them from anabolic reactions that build complex molecules. These reactions store energy in chemical bonds, which is essential for processes like photosynthesis and active transport in cells. Understanding endergonic reactions is crucial for comprehending how cells harness energy to drive metabolic activities and maintain biological functions.
Key Differences Between Anabolic and Endergonic
Anabolic processes involve the synthesis of complex molecules from simpler ones, requiring energy input, while endergonic reactions specifically describe chemical reactions that absorb energy from their surroundings. Anabolic reactions are a subset of endergonic reactions, as they build macromolecules like proteins and nucleic acids using ATP or other energy carriers. The key difference lies in scope: anabolic refers to biosynthetic pathways, whereas endergonic defines the thermodynamic energy profile of reactions.
Role of Energy in Anabolic Reactions
Anabolic reactions require an input of energy to synthesize complex molecules from simpler ones, making them endergonic processes that store energy in chemical bonds. These reactions depend on ATP hydrolysis or other energy carriers to drive the formation of macromolecules such as proteins, nucleic acids, and lipids. The energy coupling ensures that endergonic anabolic pathways proceed efficiently within cellular metabolism.
Energy Requirements of Endergonic Processes
Endergonic processes require an input of energy to proceed because they involve non-spontaneous reactions with a positive Gibbs free energy change (DG > 0). These reactions are essential in anabolic pathways, where small molecules are synthesized into complex macromolecules, consuming ATP or other energy carriers. The energy demand of endergonic reactions drives biological functions such as protein synthesis, DNA replication, and cellular growth.
Biological Examples of Anabolic Reactions
Anabolic reactions in biology synthesize complex molecules like proteins from amino acids and nucleic acids from nucleotides, requiring energy input usually from ATP hydrolysis. Photosynthesis exemplifies an anabolic process where carbon dioxide and water form glucose, storing energy in chemical bonds. These endergonic reactions drive cellular growth and repair by converting small precursors into macromolecules essential for life functions.
Biological Examples of Endergonic Reactions
Endergonic reactions in biology require energy input to proceed, with photosynthesis being a prime example, where light energy converts carbon dioxide and water into glucose and oxygen. Another key example is the synthesis of proteins from amino acids during translation, which consumes ATP to form peptide bonds. The active transport of ions across membranes, such as the sodium-potassium pump, also exemplifies endergonic processes driven by ATP hydrolysis.
Cellular Importance of Anabolic and Endergonic Reactions
Anabolic reactions are essential for cellular growth and repair, synthesizing complex molecules like proteins and nucleic acids from simpler ones. Endergonic reactions, requiring energy input, drive vital processes such as active transport and biosynthesis, ensuring cells maintain homeostasis and adapt to environmental changes. Together, these reactions enable cells to build and maintain structures, store energy, and sustain life functions.
Summary: Anabolic vs Endergonic in Metabolism
Anabolic processes in metabolism involve the synthesis of complex molecules from simpler ones, requiring an input of energy, making them inherently endergonic reactions. Endergonic reactions absorb free energy from their surroundings, driving metabolic pathways such as protein synthesis and DNA replication essential for cellular growth and repair. This energy dependence distinguishes anabolic pathways from catabolic pathways, which release energy through exergonic reactions.
Anabolic Infographic
 libterm.com
libterm.com