Hexose sugars, including glucose and fructose, play a crucial role in energy metabolism and cellular functions. These six-carbon monosaccharides are fundamental in biochemical pathways such as glycolysis and the pentose phosphate pathway. Discover how hexoses impact your health and metabolism by exploring the full article.
Table of Comparison
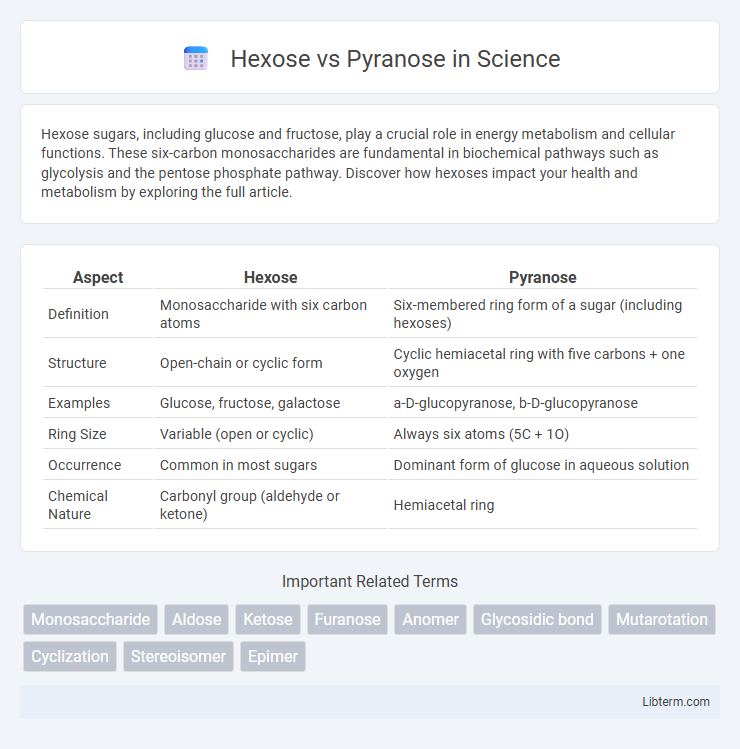
| Aspect | Hexose | Pyranose |
|---|---|---|
| Definition | Monosaccharide with six carbon atoms | Six-membered ring form of a sugar (including hexoses) |
| Structure | Open-chain or cyclic form | Cyclic hemiacetal ring with five carbons + one oxygen |
| Examples | Glucose, fructose, galactose | a-D-glucopyranose, b-D-glucopyranose |
| Ring Size | Variable (open or cyclic) | Always six atoms (5C + 1O) |
| Occurrence | Common in most sugars | Dominant form of glucose in aqueous solution |
| Chemical Nature | Carbonyl group (aldehyde or ketone) | Hemiacetal ring |
Introduction to Hexose and Pyranose
Hexoses are six-carbon monosaccharides essential in biological processes, found in forms such as glucose, fructose, and galactose. Pyranoses refer to cyclic structures of hexoses where the sugar forms a six-membered ring consisting of five carbon atoms and one oxygen atom. Understanding the distinction between hexose linear chains and pyranose ring forms is crucial in carbohydrate chemistry and metabolism.
Definition of Hexose
Hexose is a monosaccharide containing six carbon atoms, forming the backbone for important sugars such as glucose, fructose, and galactose. Pyranose refers specifically to the six-membered ring structure formed when a hexose cyclizes, resembling the chemical structure of pyran. The distinction lies in hexose defining the linear six-carbon sugar, while pyranose describes its cyclic ring form.
Definition of Pyranose
A pyranose is a six-membered cyclic sugar structure formed when a hexose undergoes intramolecular cyclization, involving five carbon atoms and one oxygen atom in the ring. Hexoses, which are monosaccharides with six carbon atoms, can exist in linear or cyclic forms, with pyranose representing the most common cyclic conformation. Pyranose rings are crucial in carbohydrate chemistry because they provide stability and influence the molecule's reactivity and interaction with biological systems.
Structural Differences: Hexose vs Pyranose
Hexoses are six-carbon monosaccharides with the general formula C6H12O6, existing primarily as linear chains or cyclic forms. Pyranoses specifically refer to hexoses in their six-membered ring structure, resembling pyran, formed through intramolecular hemiacetal formation between the carbonyl group and a hydroxyl group. The key structural difference lies in the open-chain form of hexoses versus the stable, ring-closed pyranose form, with the latter being more predominant in aqueous solutions.
Types of Hexose Sugars
Hexose sugars, six-carbon monosaccharides, exist primarily in two structural forms: pyranose and furanose, with pyranose being the most common due to its stable six-membered ring resembling pyran. Types of hexose sugars include glucose, fructose, and galactose, all of which can cyclize into pyranose forms, where glucose and galactose typically form a six-membered pyranose ring, while fructose can form both five-membered furanose and six-membered pyranose rings. The pyranose form is crucial for biological functions, such as energy metabolism and cellular recognition, because such ring structures influence the chemical reactivity and interaction of hexose sugars.
Pyranose Ring Formation
Pyranose ring formation occurs when a hexose sugar, such as glucose or galactose, undergoes intramolecular cyclization between the aldehyde or ketone group and a hydroxyl group, creating a six-membered ring structure. This hemiacetal or hemiketal formation stabilizes the sugar in its cyclic form, commonly represented as a pyranose ring due to its similarity to pyran. The ring closure significantly impacts the chemical reactivity, biological recognition, and physical properties of hexoses in biochemical pathways.
Chemical Properties Comparison
Hexose molecules, such as glucose and fructose, exhibit both open-chain and cyclic forms, with the cyclic form often existing as a pyranose ring, a six-membered oxygen-containing ring structure. Pyranoses typically display greater stability due to intramolecular hemiacetal formation, influencing their reactivity, such as resistance to oxidation compared to the open-chain form of hexoses. Chemical properties like mutarotation, solubility, and specific optical rotation differ between hexoses in their open-chain state and their pyranose ring form, impacting their biochemical roles and interactions.
Biological Significance
Hexoses, as six-carbon monosaccharides, serve as fundamental energy sources and structural components in biological systems, with glucose being the most critical for cellular respiration. Pyranoses represent a cyclic form of hexoses, typically adopting a six-membered ring that enhances molecular stability and recognition by enzymes and receptors. This cyclic structure is crucial for the proper functioning of glycoproteins, glycolipids, and energy metabolism pathways in living organisms.
Common Examples in Nature
Glucose commonly exists in nature as a pyranose ring, a six-membered structure that provides stability and facilitates energy metabolism in living organisms. Fructose, on the other hand, frequently adopts a furanose form, a five-membered ring, especially in fruits and honey, contributing to its sweetness and solubility. Mannose and galactose exemplify hexose sugars that also form pyranose rings, playing essential roles in cellular recognition and glycoprotein synthesis.
Summary and Key Distinctions
Hexose molecules are six-carbon sugars that can exist in linear or cyclic forms, with pyranose referring specifically to the six-membered ring form of a sugar. The key distinction lies in structure: hexose denotes the molecular formula C6H12O6, while pyranose defines the ring size and shape, typically a six-membered ring including five carbons and one oxygen atom. Understanding the conversion between hexose linear and pyranose cyclic forms is crucial in carbohydrate chemistry, impacting reactivity and biological function.
Hexose Infographic
 libterm.com
libterm.com