The negative terminal on a battery serves as the point where electrons exit, completing the electrical circuit. Understanding its role is crucial for safely connecting and maintaining batteries in your devices. Explore the rest of the article to learn how to handle negative terminals correctly and enhance your device's performance.
Table of Comparison
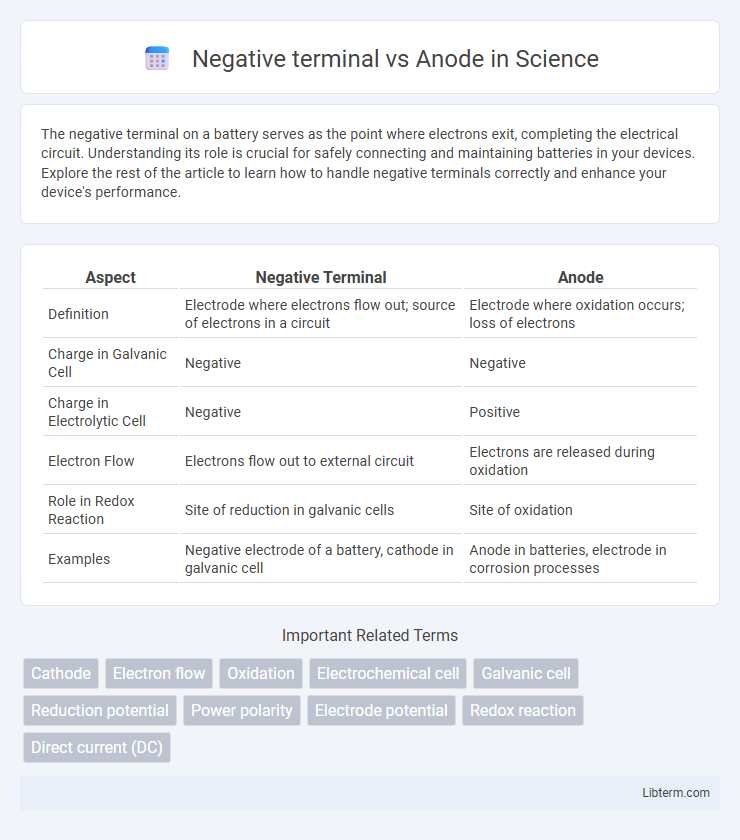
| Aspect | Negative Terminal | Anode |
|---|---|---|
| Definition | Electrode where electrons flow out; source of electrons in a circuit | Electrode where oxidation occurs; loss of electrons |
| Charge in Galvanic Cell | Negative | Negative |
| Charge in Electrolytic Cell | Negative | Positive |
| Electron Flow | Electrons flow out to external circuit | Electrons are released during oxidation |
| Role in Redox Reaction | Site of reduction in galvanic cells | Site of oxidation |
| Examples | Negative electrode of a battery, cathode in galvanic cell | Anode in batteries, electrode in corrosion processes |
Understanding Negative Terminal and Anode: Core Concepts
The negative terminal in electrical circuits refers to the point where electrons flow out of a power source, such as a battery, often marked as the cathode during discharge. The anode, in electrochemical cells, is the electrode where oxidation occurs and electrons are released, which can be either negative or positive depending on the type of cell. Understanding the distinction hinges on recognizing that the negative terminal is defined by electron flow in the circuit, while the anode's charge varies with cell operation, emphasizing their functional roles in energy transfer.
Key Differences Between Negative Terminal and Anode
The negative terminal of a battery refers to the electrode where electrons flow out during discharge, typically marked as the source of electrical current in a circuit. The anode is defined as the electrode where oxidation occurs, releasing electrons into the external circuit; in galvanic cells, the anode corresponds to the negative terminal, while in electrolytic cells, it is positive. Key differences include the negative terminal being a polarity designation in electrical devices, whereas the anode is a functional term describing the site of electron loss through chemical reactions within electrochemical cells.
Roles in Electrical Circuits
The negative terminal in electrical circuits serves as the source of electrons, providing the flow of current toward the positive terminal. The anode is the electrode where oxidation occurs, typically acting as the point where current leaves in galvanic cells but enters in electrolytic cells. Understanding the specific role of the negative terminal and anode is crucial for analyzing circuit behavior and electrochemical reactions accurately.
Negative Terminal in Batteries and Electronic Devices
The negative terminal in batteries serves as the source of electrons, facilitating the flow of current through an external circuit during discharge. Unlike the anode, which is defined by the direction of current flow in electrochemical reactions, the negative terminal consistently provides the electrical connection where electrons exit the battery. This distinction is crucial in electronic devices, as the negative terminal ensures proper polarity and functionality of the circuit.
Anode Functionality in Various Applications
The anode serves as the electrode where oxidation occurs, playing a crucial role in electrochemical cells, corrosion protection, and semiconductor technology. In batteries, the anode releases electrons to the external circuit, enabling electrical flow during discharge, while in electrolytic cells, it attracts anions and facilitates oxidation reactions. Its specific functionality varies by application, such as in cathodic protection systems where the anode sacrifices itself to prevent metal corrosion or in LED devices where it manages hole injection.
Common Misconceptions: Negative Terminal vs Anode
The negative terminal of a battery is often confused with the anode, but they are not always the same; in a discharging battery, the anode is the negative terminal where oxidation occurs, while in electrolytic cells, the anode is positive due to the direction of current flow. Misunderstanding arises because the negative terminal is defined by electrical polarity, whereas the anode is defined by the site of oxidation, which varies between galvanic and electrolytic cells. Clarifying that the anode's charge depends on the cell type helps eliminate common misconceptions in electrochemistry and battery terminal identification.
Importance in Electrochemical Cells
The negative terminal in an electrochemical cell, commonly known as the anode in galvanic cells, is where oxidation occurs and electrons are released into the external circuit. Understanding the anode's role is crucial because it drives the flow of electrons, enabling the cell to generate electrical energy efficiently. Distinguishing between the negative terminal and the anode is vital for properly designing and optimizing batteries and other electrochemical devices.
Impact on Device Performance and Safety
The negative terminal and anode play crucial roles in device performance and safety, with the negative terminal typically serving as the source of electrons in most batteries, while the anode's function varies depending on the device type, such as in electrolytic cells where it is the positive electrode. Incorrect identification or connection of these terminals can lead to reduced efficiency, increased heat generation, and potential device failure. Safety is also impacted as improper handling or polarity reversal may cause short circuits, chemical leaks, or even explosions, highlighting the importance of accurate terminal usage and design in electronic and electrochemical systems.
Practical Examples: Identifying Each in Real Devices
In a typical alkaline battery, the negative terminal serves as the cathode during discharge, while the anode is the positive terminal, which might confuse those familiar with electrochemical cell conventions. In devices like LEDs or diodes, the anode is identified as the terminal where current enters, marked by the longer lead, whereas the negative terminal is usually connected to the device's ground or common return path. For rechargeable lithium-ion cells, the anode is generally the negative electrode during discharge, often made of graphite, contrasting with primary cells, highlighting the importance of device-specific context when identifying terminals.
Summary Table: Negative Terminal vs Anode
The negative terminal is the point in a battery or electrical circuit where electrons enter, typically marked as the source of current flow in external circuits. The anode, defined by its role in electrochemical reactions, is the electrode where oxidation occurs, often corresponding to the negative terminal in galvanic cells but positive in electrolytic cells. Summary tables comparing the negative terminal and anode highlight distinctions based on circuit function, electrode reactions, and cell type to clarify their roles in energy conversion and electron flow.
Negative terminal Infographic
 libterm.com
libterm.com