Isochoric processes maintain constant volume, meaning the system's spatial dimensions do not change during the transformation. This concept is crucial in thermodynamics, particularly when analyzing pressure and temperature variations without volume alteration. Explore the rest of the article to understand how isochoric conditions impact different physical systems and processes.
Table of Comparison
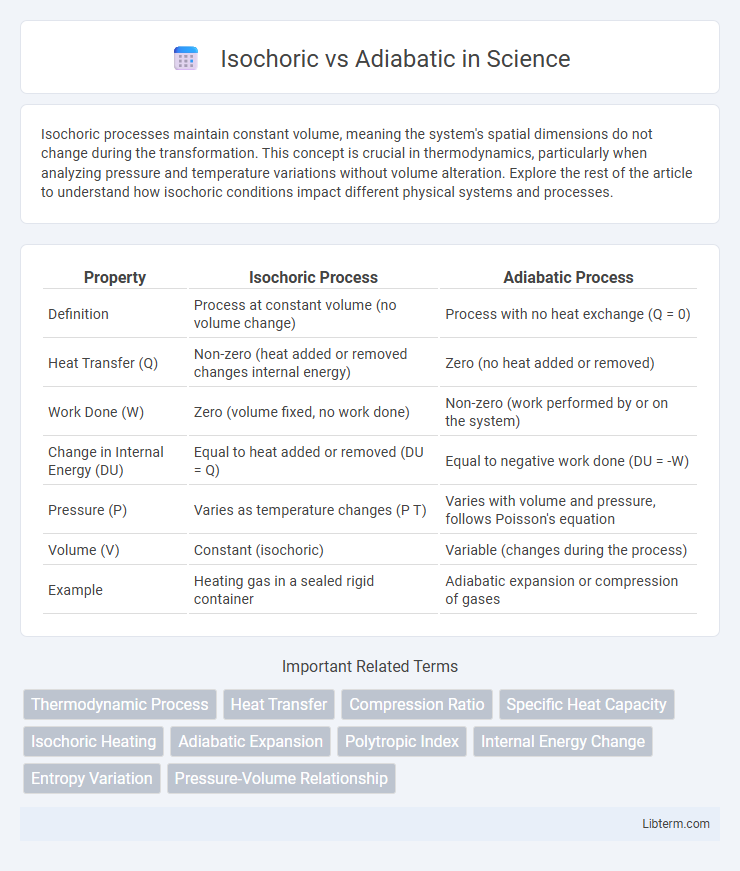
| Property | Isochoric Process | Adiabatic Process |
|---|---|---|
| Definition | Process at constant volume (no volume change) | Process with no heat exchange (Q = 0) |
| Heat Transfer (Q) | Non-zero (heat added or removed changes internal energy) | Zero (no heat added or removed) |
| Work Done (W) | Zero (volume fixed, no work done) | Non-zero (work performed by or on the system) |
| Change in Internal Energy (DU) | Equal to heat added or removed (DU = Q) | Equal to negative work done (DU = -W) |
| Pressure (P) | Varies as temperature changes (P T) | Varies with volume and pressure, follows Poisson's equation |
| Volume (V) | Constant (isochoric) | Variable (changes during the process) |
| Example | Heating gas in a sealed rigid container | Adiabatic expansion or compression of gases |
Understanding Isochoric and Adiabatic Processes
Isochoric and adiabatic processes represent fundamental thermodynamic transformations where volume and heat exchange differ significantly. An isochoric process occurs at constant volume, meaning no work is done by the system as pressure and temperature change, while an adiabatic process involves no heat transfer with the environment, causing temperature and pressure to change as the system does work on or by the surroundings. Understanding these processes requires analyzing their impact on internal energy, pressure, temperature, and the work done in thermodynamic systems, critical for applications like engine cycles and HVAC systems.
Basic Thermodynamic Principles
Isochoric processes maintain constant volume, resulting in no work done by the system as pressure and temperature change. Adiabatic processes occur without heat exchange, causing internal energy shifts to directly convert into work, changing both pressure and volume. Understanding these principles is crucial for analyzing energy transfer and system behavior in thermodynamic cycles.
Defining Isochoric Processes
Isochoric processes occur at a constant volume, meaning no work is done as the system's volume does not change. In thermodynamics, the pressure and temperature of the gas vary while the volume remains fixed, often seen in rigid containers. This contrasts with adiabatic processes, where no heat exchange occurs but volume and pressure typically change.
Characteristics of Adiabatic Processes
Adiabatic processes are characterized by the absence of heat exchange with the environment, meaning that all energy changes manifest solely as work done by or on the system. During these processes, the internal energy variation corresponds directly to changes in temperature and pressure without heat transfer, often resulting in rapid compression or expansion in gases. This contrasts with isochoric processes where volume remains constant, emphasizing that adiabatic processes involve dynamic volume and pressure variations under thermally isolated conditions.
Key Differences: Isochoric vs Adiabatic
Isochoric processes occur at constant volume, meaning no work is done on or by the system, and pressure changes result solely from temperature variations. Adiabatic processes involve no heat exchange with the surroundings, causing temperature and pressure changes due to work done by or on the system as volume changes. Key differences include the constraints: isochoric processes maintain fixed volume, while adiabatic processes allow volume changes without heat transfer.
Pressure-Volume Relationships
Isochoric processes maintain constant volume, causing pressure to change directly with temperature according to Gay-Lussac's law, reflecting a vertical line on a pressure-volume (P-V) diagram. Adiabatic processes involve no heat exchange, with pressure and volume linked by \( PV^\gamma = \text{constant} \), where \( \gamma \) is the heat capacity ratio, producing a steeper curve on the P-V diagram than isochoric or isothermal processes. Understanding the distinct pressure-volume relationships in isochoric and adiabatic processes is crucial for thermodynamic cycle analysis and engineering applications such as internal combustion engines and refrigeration systems.
Impact on Temperature and Internal Energy
Isochoric processes maintain constant volume, causing all heat added to increase the internal energy and temperature of the system directly. Adiabatic processes involve no heat exchange, so changes in internal energy result solely from work done by or on the system, leading to temperature changes without heat transfer. The distinction impacts thermodynamic calculations, with isochoric processes showing direct proportionality between heat input and internal energy increase, while adiabatic processes rely on work interactions to alter temperature and internal energy.
Practical Applications in Engineering
Isochoric processes, characterized by constant volume, are crucial in applications like internal combustion engines where volume remains fixed during heat transfer, enabling efficient pressure control. Adiabatic processes, involving no heat exchange, are essential in gas turbine and compressor design to maximize energy conversion by minimizing thermal losses. Engineers leverage isochoric and adiabatic principles to optimize thermodynamic cycles for improved engine performance and energy efficiency.
Graphical Representation (P-V Diagrams)
Isochoric processes appear as vertical lines on P-V diagrams, indicating constant volume with pressure changes, while adiabatic processes show steep, curved lines representing simultaneous pressure and volume changes without heat exchange. The slope of an adiabatic curve is steeper than an isothermal curve due to the absence of heat transfer, reflecting changes in internal energy. Isochoric lines do not move horizontally because volume remains fixed, clearly distinguishing them from adiabatic curves on the graph.
Summary and Comparative Analysis
Isochoric processes occur at constant volume with no work done by the system, while adiabatic processes involve no heat exchange with the surroundings, resulting in temperature and pressure changes due to work done. In isochoric processes, internal energy changes solely affect temperature, whereas in adiabatic processes, energy changes manifest through pressure-volume work without heat transfer. Thermodynamic analysis highlights that isochoric processes feature vertical lines on the PV diagram, in contrast to the curved trajectories of adiabatic processes, emphasizing distinct energy transfer mechanisms.
Isochoric Infographic
 libterm.com
libterm.com