Mass spectrometry precisely identifies and quantifies molecules by measuring their mass-to-charge ratio, offering critical insights in fields like pharmaceuticals, environmental analysis, and proteomics. Its sensitivity and accuracy enable the detection of trace compounds, making it indispensable for complex mixture analysis. Explore the rest of the article to understand how mass spectrometry can enhance your scientific research and analytical capabilities.
Table of Comparison
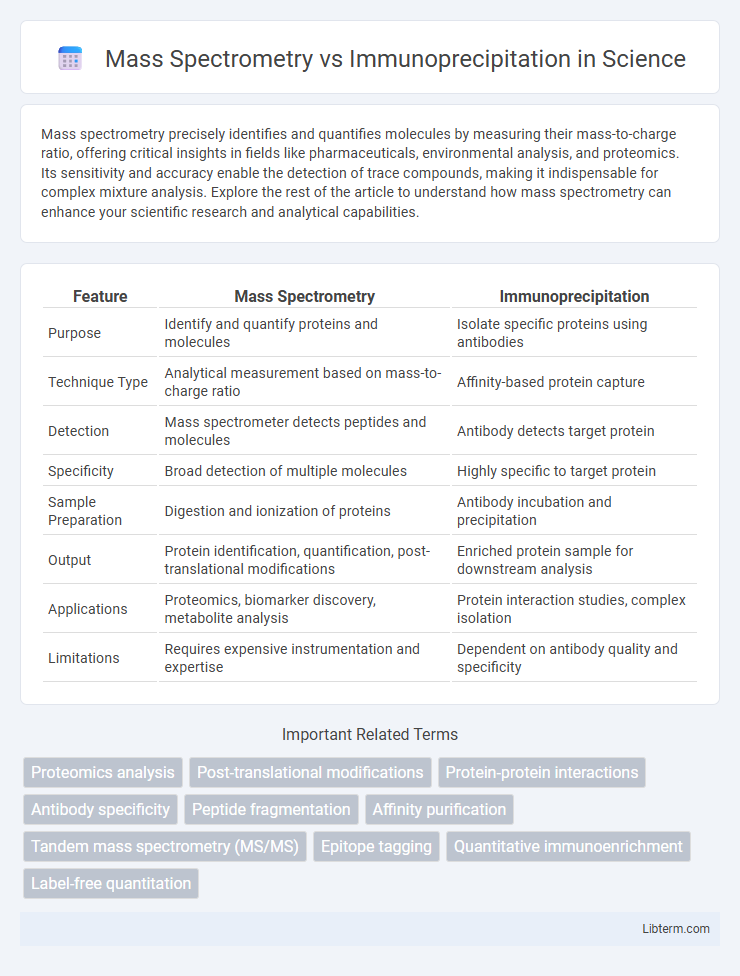
| Feature | Mass Spectrometry | Immunoprecipitation |
|---|---|---|
| Purpose | Identify and quantify proteins and molecules | Isolate specific proteins using antibodies |
| Technique Type | Analytical measurement based on mass-to-charge ratio | Affinity-based protein capture |
| Detection | Mass spectrometer detects peptides and molecules | Antibody detects target protein |
| Specificity | Broad detection of multiple molecules | Highly specific to target protein |
| Sample Preparation | Digestion and ionization of proteins | Antibody incubation and precipitation |
| Output | Protein identification, quantification, post-translational modifications | Enriched protein sample for downstream analysis |
| Applications | Proteomics, biomarker discovery, metabolite analysis | Protein interaction studies, complex isolation |
| Limitations | Requires expensive instrumentation and expertise | Dependent on antibody quality and specificity |
Introduction to Mass Spectrometry and Immunoprecipitation
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of ions, enabling precise identification and quantification of proteins, peptides, and other biomolecules in complex mixtures. Immunoprecipitation employs specific antibodies to isolate and concentrate a target protein from a complex sample, facilitating downstream analysis of protein-protein interactions or post-translational modifications. Both techniques are essential in proteomics, where mass spectrometry offers high-throughput molecular characterization, and immunoprecipitation provides selective enrichment of proteins of interest.
Principle of Mass Spectrometry
Mass spectrometry operates on the principle of ionizing chemical compounds to generate charged molecules or molecule fragments and measuring their mass-to-charge ratios using electromagnetic fields. This technique enables precise identification and quantification of proteins, peptides, and other biomolecules based on their unique mass spectral signatures. In contrast, immunoprecipitation relies on antibody-antigen specificity to isolate target proteins from complex mixtures but lacks the molecular weight profiling inherent to mass spectrometry.
Principle of Immunoprecipitation
Immunoprecipitation is a technique that isolates a specific antigen from a complex mixture using an antibody that specifically binds to that target protein, forming an antibody-antigen complex. This complex is then captured by protein A/G beads, allowing for precipitation and separation by centrifugation. Mass spectrometry can subsequently analyze the purified proteins, providing detailed information on molecular weight, peptide sequences, and post-translational modifications.
Applications of Mass Spectrometry in Proteomics
Mass spectrometry in proteomics enables precise identification and quantification of proteins, facilitating the analysis of complex biological samples at high throughput. It allows for the detection of post-translational modifications, protein-protein interactions, and protein isoforms, enhancing the understanding of cellular functions and disease mechanisms. In contrast to immunoprecipitation, which isolates specific protein complexes, mass spectrometry provides a comprehensive proteome-wide analysis.
Applications of Immunoprecipitation in Protein Analysis
Immunoprecipitation is primarily used for isolating and concentrating specific proteins from complex biological samples, enabling detailed analysis of protein-protein interactions, post-translational modifications, and protein complexes. This technique is invaluable in characterizing signaling pathways, identifying binding partners, and validating antibody specificity. While mass spectrometry provides comprehensive protein identification and quantification, immunoprecipitation offers targeted enrichment necessary for subsequent mass spectrometry or western blot analysis.
Sensitivity and Specificity Comparison
Mass spectrometry offers high sensitivity by detecting low-abundance proteins through precise mass-to-charge ratio measurements, enabling identification of complex protein mixtures with minimal background noise. Immunoprecipitation provides high specificity by using antibodies to selectively isolate target proteins or protein complexes, but its sensitivity depends on antibody affinity and expression levels of the target. Combining mass spectrometry with immunoprecipitation enhances both sensitivity and specificity, allowing detailed analysis of protein interactions and post-translational modifications.
Strengths and Limitations of Mass Spectrometry
Mass spectrometry offers high sensitivity and accuracy in identifying and quantifying proteins, enabling comprehensive analysis of complex biological samples and post-translational modifications. Its limitations include high operational costs, requirement for extensive sample preparation, and dependence on sophisticated data analysis tools. Compared to immunoprecipitation, mass spectrometry provides unbiased protein identification but may lack the specificity and simplicity of antibody-based target enrichment.
Strengths and Limitations of Immunoprecipitation
Immunoprecipitation excels at isolating specific proteins from complex mixtures using targeted antibodies, allowing detailed analysis of protein-protein interactions and post-translational modifications. However, its limitations include dependence on antibody specificity and affinity, potential for non-specific binding, and lower throughput compared to mass spectrometry. Despite these constraints, immunoprecipitation remains invaluable for validating protein complexes prior to comprehensive mass spectrometric analysis.
Workflow Differences: Sample Preparation and Analysis
Mass spectrometry involves complex sample preparation including protein extraction, digestion into peptides, and often peptide fractionation before analysis, enabling highly sensitive and quantitative identification of proteins. Immunoprecipitation requires antibody binding to specifically capture target proteins or complexes directly from cell lysates, followed by washing steps to reduce nonspecific binding prior to downstream analysis. Unlike immunoprecipitation, mass spectrometry workflows emphasize in-depth proteome coverage without reliance on antibodies, while immunoprecipitation provides targeted enrichment suited for analyzing protein-protein interactions or post-translational modifications.
Choosing the Right Technique: Factors to Consider
Choosing between Mass Spectrometry and Immunoprecipitation depends on the experimental goals, sample complexity, and target specificity. Mass Spectrometry offers high-throughput identification and quantification of proteins in complex mixtures, ideal for discovery-based studies. Immunoprecipitation provides high specificity for isolating protein complexes or post-translationally modified proteins, making it suitable when targeting specific protein interactions or modifications.
Mass Spectrometry Infographic
 libterm.com
libterm.com