Thermal technology plays a crucial role in managing heat transfer across various applications, from electronics cooling to energy-efficient building designs. Understanding thermal properties can significantly enhance the performance and durability of your devices and infrastructure. Explore the rest of the article to discover how optimizing thermal solutions can benefit your projects.
Table of Comparison
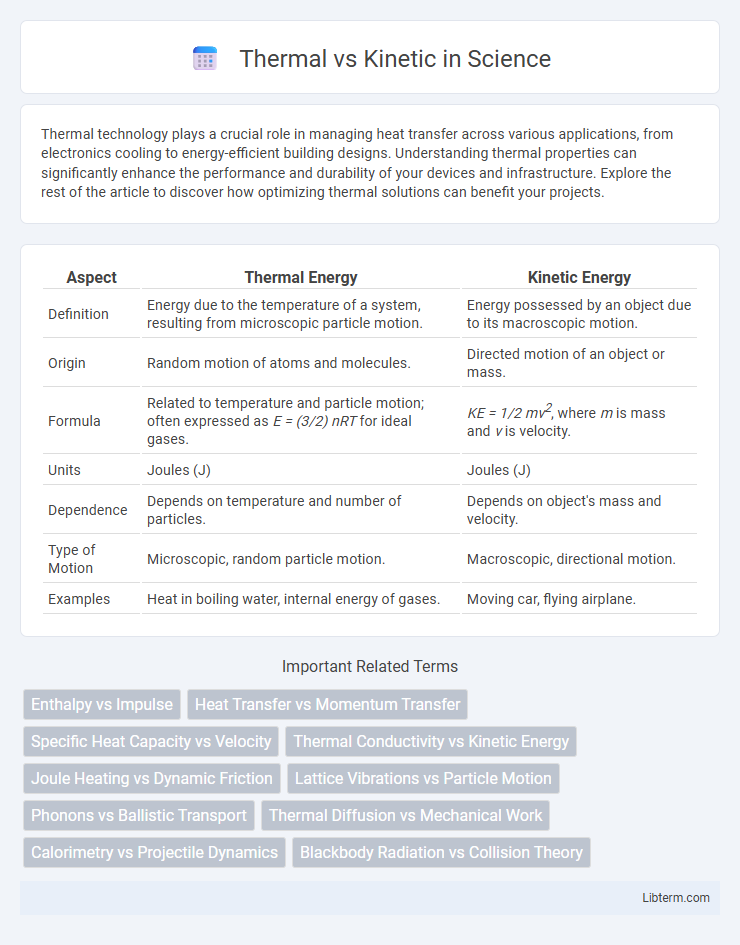
| Aspect | Thermal Energy | Kinetic Energy |
|---|---|---|
| Definition | Energy due to the temperature of a system, resulting from microscopic particle motion. | Energy possessed by an object due to its macroscopic motion. |
| Origin | Random motion of atoms and molecules. | Directed motion of an object or mass. |
| Formula | Related to temperature and particle motion; often expressed as E = (3/2) nRT for ideal gases. | KE = 1/2 mv2, where m is mass and v is velocity. |
| Units | Joules (J) | Joules (J) |
| Dependence | Depends on temperature and number of particles. | Depends on object's mass and velocity. |
| Type of Motion | Microscopic, random particle motion. | Macroscopic, directional motion. |
| Examples | Heat in boiling water, internal energy of gases. | Moving car, flying airplane. |
Introduction to Thermal and Kinetic Energy
Thermal energy refers to the internal energy present in a system due to the random motion of its particles, directly proportional to temperature. Kinetic energy is the energy an object possesses due to its motion, calculated as half the mass times the velocity squared. Understanding the distinction between thermal and kinetic energy is crucial for applications in thermodynamics and mechanics.
Defining Thermal Energy
Thermal energy refers to the internal energy present in a system due to the random motion of its particles, directly related to temperature and heat transfer. Kinetic energy, on the other hand, is the energy an object possesses due to its macroscopic motion or velocity. While kinetic energy measures the energy of movement at a larger scale, thermal energy reflects microscopic particle vibrations and interactions within matter.
Understanding Kinetic Energy
Kinetic energy is the energy possessed by an object due to its motion, calculated as half the mass times the velocity squared (KE = 1/2 mv2), making it a fundamental concept in classical mechanics. Unlike thermal energy, which is the total microscopic energy in a system arising from random particle motion, kinetic energy specifically refers to the macroscopic, organized movement of an object. Understanding kinetic energy helps explain phenomena ranging from vehicle dynamics to ballistic trajectories and molecular movement in gases.
Key Differences Between Thermal and Kinetic Energy
Thermal energy refers to the total internal energy present in a system due to the random motion of its molecules, while kinetic energy is the energy possessed by an object due to its motion as a whole. Thermal energy depends on temperature and molecular interactions within a substance, whereas kinetic energy depends on the mass and velocity of a moving object. Key differences include the scale of motion--microscopic for thermal energy versus macroscopic for kinetic energy--and the nature of energy transfer, with thermal energy involving heat exchange and kinetic energy involving mechanical work.
How Thermal Energy is Generated
Thermal energy is generated through the transfer of kinetic energy from rapidly moving particles within a substance, causing increased vibrations and collisions at the atomic or molecular level. This transfer can occur via conduction, convection, or radiation, where heat moves from a higher temperature region to a lower one. The microscopic motion of atoms and molecules translates kinetic energy into thermal energy, raising the temperature of the material.
Sources of Kinetic Energy
Kinetic energy originates from the motion of objects, such as moving vehicles, flowing water, and wind, which harness mechanical forces to generate power. Sources of kinetic energy include wind turbines converting natural air flow and hydroelectric plants utilizing river currents to produce electricity. Human activity also contributes through muscular motion and machinery operation, emphasizing the direct transformation of movement into usable energy.
Applications of Thermal Energy in Daily Life
Thermal energy powers household heating systems, cooking appliances, and water heaters, enabling comfort and food preparation through controlled heat transfer. Solar thermal panels convert sunlight into usable heat for space heating and hot water, increasing energy efficiency in residential and commercial buildings. Industrial processes utilize thermal energy for manufacturing tasks such as metal forging, chemical reactions, and sterilization, highlighting its vital role in everyday applications.
Practical Uses of Kinetic Energy
Kinetic energy powers everyday devices such as wind turbines, converting wind motion into electricity, and hydroelectric plants that utilize flowing water to generate power. Transportation systems, including cars, trains, and bicycles, rely on kinetic energy for movement and fuel efficiency. Industrial applications harness kinetic energy through machinery like conveyor belts and turbines, optimizing productivity and energy consumption.
Thermal vs Kinetic Energy in Industry
Thermal energy in industry primarily involves heat transfer processes such as steam generation, heating, and combustion to power machinery or chemical reactions, optimizing production efficiency. Kinetic energy impacts industrial applications through the motion of fluids, rotating equipment, and conveyor systems, playing a crucial role in mechanical operations and transportation of materials. Balancing thermal and kinetic energy management enhances energy utilization and reduces operational costs across manufacturing sectors.
Choosing Between Thermal and Kinetic Energy
Choosing between thermal and kinetic energy depends on the application's efficiency and purpose; kinetic energy is ideal for mechanical work and motion-driven systems, while thermal energy suits processes requiring heat transfer or temperature control. Thermal energy, measured in joules or calories, often involves the random motion of particles, whereas kinetic energy specifically relates to the organized movement of objects with mass. Selecting the right form involves analyzing energy conversion efficiency, system design, and the desired outcome, such as power generation, heating, or propulsion.
Thermal Infographic
 libterm.com
libterm.com