Conformers are different spatial arrangements of a molecule that result from rotation around single bonds, significantly impacting chemical properties and reactivity. These three-dimensional shapes influence how molecules interact in biological systems, affecting drug design and protein folding. Discover how understanding conformers can enhance your grasp of molecular behavior in the detailed insights ahead.
Table of Comparison
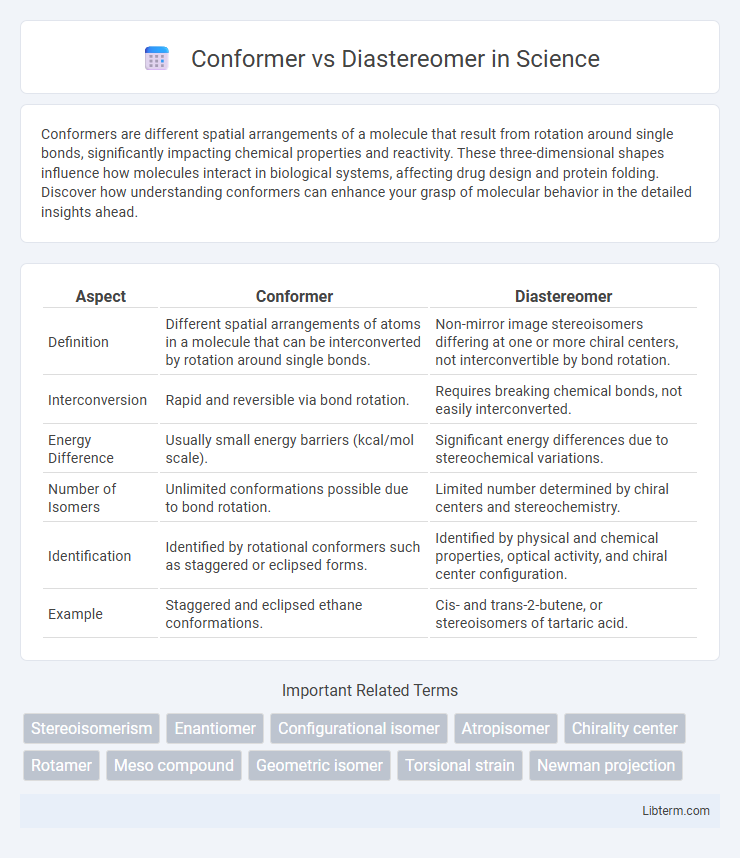
| Aspect | Conformer | Diastereomer |
|---|---|---|
| Definition | Different spatial arrangements of atoms in a molecule that can be interconverted by rotation around single bonds. | Non-mirror image stereoisomers differing at one or more chiral centers, not interconvertible by bond rotation. |
| Interconversion | Rapid and reversible via bond rotation. | Requires breaking chemical bonds, not easily interconverted. |
| Energy Difference | Usually small energy barriers (kcal/mol scale). | Significant energy differences due to stereochemical variations. |
| Number of Isomers | Unlimited conformations possible due to bond rotation. | Limited number determined by chiral centers and stereochemistry. |
| Identification | Identified by rotational conformers such as staggered or eclipsed forms. | Identified by physical and chemical properties, optical activity, and chiral center configuration. |
| Example | Staggered and eclipsed ethane conformations. | Cis- and trans-2-butene, or stereoisomers of tartaric acid. |
Introduction to Conformers and Diastereomers
Conformers are different spatial arrangements of a molecule that result from rotation around single bonds, allowing the molecule to adopt multiple energetically favorable shapes without breaking covalent bonds. Diastereomers are stereoisomers that are not mirror images of each other and have different physical and chemical properties due to the presence of multiple chiral centers. Understanding the distinction between conformers and diastereomers is essential for analyzing stereochemistry and predicting molecular behavior in chemical reactions.
Defining Conformers: Structure and Characteristics
Conformers are distinct spatial arrangements of a molecule that interconvert through rotation around single bonds without breaking covalent bonds, defining their dynamic nature. These structures often differ in energy levels and steric interactions, influencing molecular properties such as reactivity and stability. Unlike diastereomers, conformers do not involve changes in stereochemistry but represent different molecular shapes within the same stereoisomer.
Understanding Diastereomers: Key Features
Diastereomers are stereoisomers with multiple chiral centers that differ in configuration at one or more, but not all, stereocenters, resulting in distinct physical and chemical properties unlike conformers, which are rotational isomers of the same molecule. Key features of diastereomers include different melting points, boiling points, and optical activities, allowing separation through conventional methods such as crystallization or chromatography. Unlike conformers, diastereomers are not interconvertible by simple bond rotations, making them a critical concept in stereochemistry and pharmaceutical design.
Structural Differences Between Conformers and Diastereomers
Conformers differ in spatial arrangement due to rotation around single bonds without breaking any bonds, resulting in dynamic structures like staggered or eclipsed forms. Diastereomers have distinct configurations at one or more stereocenters and are non-superimposable, non-mirror-image stereoisomers with different physical and chemical properties. Structural differences arise because conformers interconvert rapidly through bond rotations, whereas diastereomers require bond-breaking to interconvert.
Importance in Organic Chemistry
Conformers and diastereomers are crucial in organic chemistry for understanding molecular behavior and stereochemistry. Conformers represent different spatial arrangements of atoms due to rotation around single bonds, influencing reaction mechanisms and physical properties without breaking bonds. Diastereomers, as stereoisomers that are not mirror images, affect chemical reactivity and biological activity, playing a key role in drug design and synthesis.
Methods for Identification and Analysis
Conformers are identified and analyzed primarily through techniques like Nuclear Magnetic Resonance (NMR) spectroscopy, which provides dynamic information about molecular rotations and energy barriers. Diastereomers are distinguished using methods such as High-Performance Liquid Chromatography (HPLC) and X-ray crystallography, which offer detailed stereochemical and structural insights. Computational chemistry tools, including molecular modeling and density functional theory (DFT), complement experimental approaches by predicting conformer stability and diastereomeric configurations.
Physical and Chemical Properties Comparison
Conformers exhibit dynamic interconversion through bond rotation without breaking covalent bonds, resulting in similar physical properties such as melting point and solubility, whereas diastereomers have distinct stereochemistry that leads to different physical properties including varied melting points, boiling points, and specific optical rotations. Chemically, conformers generally show comparable reactivity under identical conditions, but diastereomers often display divergent chemical reactivity and biological activity due to their stereochemical differences. The energy barrier between conformers dictates conformational preferences, while the fixed stereochemistry of diastereomers results in more pronounced differences in both chemical and physical behavior.
Role in Stereochemical Reactions
Conformers influence stereochemical reactions by affecting the spatial arrangement of atoms, thereby determining reaction pathways and product stereochemistry without breaking covalent bonds. Diastereomers, possessing different stereochemical configurations, exhibit distinct chemical and physical properties, leading to varied reactivity and selectivity in stereochemical transformations. Understanding the interplay between conformational flexibility and diastereomeric differences is crucial for controlling stereochemical outcomes in organic synthesis.
Applications in Pharmaceuticals and Material Science
Conformers, which are different spatial arrangements of a molecule due to rotation around single bonds, are crucial in pharmaceuticals for optimizing drug-receptor interactions and enhancing bioavailability. Diastereomers, stereoisomers that are not mirror images, exhibit distinct physical and chemical properties, making them essential in drug design for specific therapeutic effects and reduced side effects. In material science, conformers influence polymer flexibility and crystallinity, while diastereomers contribute to the development of chiral materials with unique optical and mechanical properties.
Summary: Choosing Conformer or Diastereomer Analysis
Choosing between conformer and diastereomer analysis depends on the molecular flexibility and stereochemical differences involved. Conformer analysis focuses on the spatial arrangement resulting from rotations around single bonds, crucial for understanding dynamic molecular behavior. Diastereomer analysis examines stereoisomers with different configurations at one or more chiral centers, important for distinguishing discrete stereochemical entities with different physical and chemical properties.
Conformer Infographic
 libterm.com
libterm.com