Polar regions are characterized by extreme cold temperatures, unique ecosystems, and vast ice-covered landscapes that play a crucial role in Earth's climate system. Understanding the dynamics of polar ice caps and their impact on sea level rise is essential for addressing global environmental challenges. Discover how these icy frontiers influence our planet and what it means for Your future by reading the rest of the article.
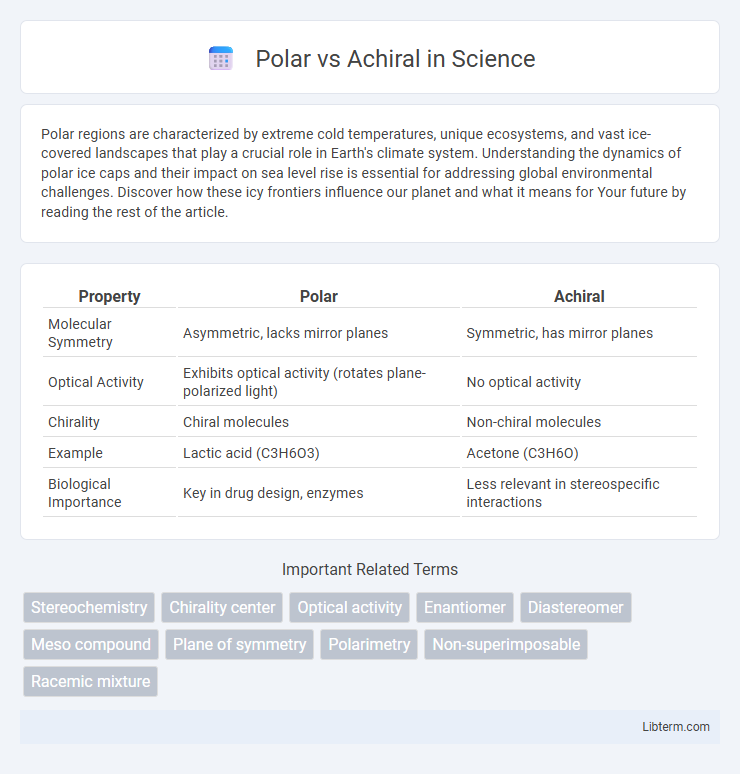
Table of Comparison
| Property | Polar | Achiral |
|---|---|---|
| Molecular Symmetry | Asymmetric, lacks mirror planes | Symmetric, has mirror planes |
| Optical Activity | Exhibits optical activity (rotates plane-polarized light) | No optical activity |
| Chirality | Chiral molecules | Non-chiral molecules |
| Example | Lactic acid (C3H6O3) | Acetone (C3H6O) |
| Biological Importance | Key in drug design, enzymes | Less relevant in stereospecific interactions |
Introduction to Polar and Achiral Compounds
Polar compounds contain molecules with uneven charge distribution resulting in distinct positive and negative poles, which influence their solubility and reactivity. Achiral compounds lack chirality, meaning they have no handedness and are superimposable on their mirror images, affecting their interaction with polarized light and biological systems. Understanding the difference between polar and achiral substances is essential in fields such as chemistry and pharmacology for predicting molecular behavior and designing effective compounds.
Understanding Molecular Polarity
Polar molecules possess an uneven distribution of electron density, resulting in partial positive and negative charges that create a dipole moment. Achiral molecules, lacking a chiral center, can be either polar or nonpolar depending on their molecular geometry and the electronegativity of their atoms. Understanding molecular polarity requires analyzing both molecular shape and bond dipoles to determine whether the dipole moments cancel out or reinforce each other.
What Does Achiral Mean in Chemistry?
Achiral refers to molecules that are superimposable on their mirror images and lack chirality or "handedness." Unlike polar molecules, which have an uneven distribution of electron density resulting in dipole moments, achiral molecules do not exhibit optical activity or enantiomerism. Common examples of achiral molecules include ethane and benzene, which possess symmetry elements such as planes of symmetry or centers of inversion.
Key Differences Between Polar and Achiral Molecules
Polar molecules possess an uneven distribution of electron density resulting in a permanent dipole moment, whereas achiral molecules lack handedness and are superimposable on their mirror images. Polar molecules exhibit dipole-dipole interactions influencing properties like solubility and boiling point, while achiral molecules do not show optical activity and cannot rotate plane-polarized light. The key structural difference lies in polar molecules having regions of partial positive and negative charge, whereas achiral molecules have symmetrical molecular geometry that prevents chirality.
Structural Features of Polar Compounds
Polar compounds exhibit asymmetric molecular structures with uneven distribution of electron density, leading to a permanent dipole moment, unlike achiral molecules which are often symmetric and lack such dipole moments. Functional groups such as hydroxyl (-OH), carbonyl (C=O), and amine (-NH2) contribute to polarity by creating regions of partial positive and negative charges within the molecule. Structural features like bent or angular geometries in molecules (e.g., water) reinforce polarity, whereas achiral molecules typically have symmetrical arrangements that cancel out dipole moments, resulting in nonpolar characteristics.
Characteristics of Achiral Molecules
Achiral molecules lack a plane of symmetry and do not have non-superimposable mirror images, resulting in identical enantiomers. These molecules exhibit no optical activity because they cannot rotate plane-polarized light. Common characteristics include the presence of symmetrical structures and the absence of chiral centers, which distinguishes them from polar, chiral counterparts.
Polarity and Chirality: Are They Related?
Polar molecules have an uneven distribution of electron density, resulting in a dipole moment, while achiral molecules lack chirality, meaning they are superimposable on their mirror images. Polarity and chirality describe different molecular properties; polarity involves electron distribution and dipole moments, whereas chirality relates to spatial arrangement and stereochemistry. A molecule can be polar without being chiral, and achiral molecules can be either polar or nonpolar depending on their structure.
Examples of Polar vs. Achiral Substances
Water (H2O) exemplifies a polar substance due to its bent molecular shape and uneven charge distribution, resulting in a permanent dipole moment. Carbon tetrachloride (CCl4) serves as a classic achiral molecule with a symmetric tetrahedral geometry, evenly distributing electron density and lacking optical activity. Ammonia (NH3) is another polar molecule with a trigonal pyramidal shape causing molecular polarity, while methane (CH4) depicts an achiral compound with a tetrahedral, symmetric structure that cancels out any dipole moments.
Applications in Industry and Research
Polar molecules exhibit distinct dipole moments that enhance their solvent properties and reactivity, making them essential in pharmaceuticals for drug design and in materials science for polymer synthesis. Achiral compounds, lacking optical activity, are crucial in producing racemic mixtures used in industrial catalysis and flavor chemicals where uniformity is desired. Both molecular types enable tailored applications in chemical manufacturing, analytical chemistry, and nanotechnology research through their unique interactions and stereochemical properties.
Summary: Importance of Distinguishing Polar and Achiral
Distinguishing between polar and achiral molecules is crucial in fields such as pharmaceuticals and materials science, where molecular interactions and properties vary significantly based on polarity and chirality. Polar molecules exhibit uneven charge distribution, affecting solubility and reactivity, while achiral molecules lack handedness, simplifying stereochemical analysis. Understanding these differences enhances drug design, enantioselective synthesis, and the development of functional materials with desired chemical behaviors.
Polar Infographic
 libterm.com
libterm.com