Photolysis is a chemical process where light energy breaks down molecules, often playing a vital role in atmospheric reactions and biological systems. This mechanism drives the decomposition of pollutants and contributes to the formation of essential compounds like ozone. Explore the detailed dynamics and applications of photolysis in the rest of the article.
Table of Comparison
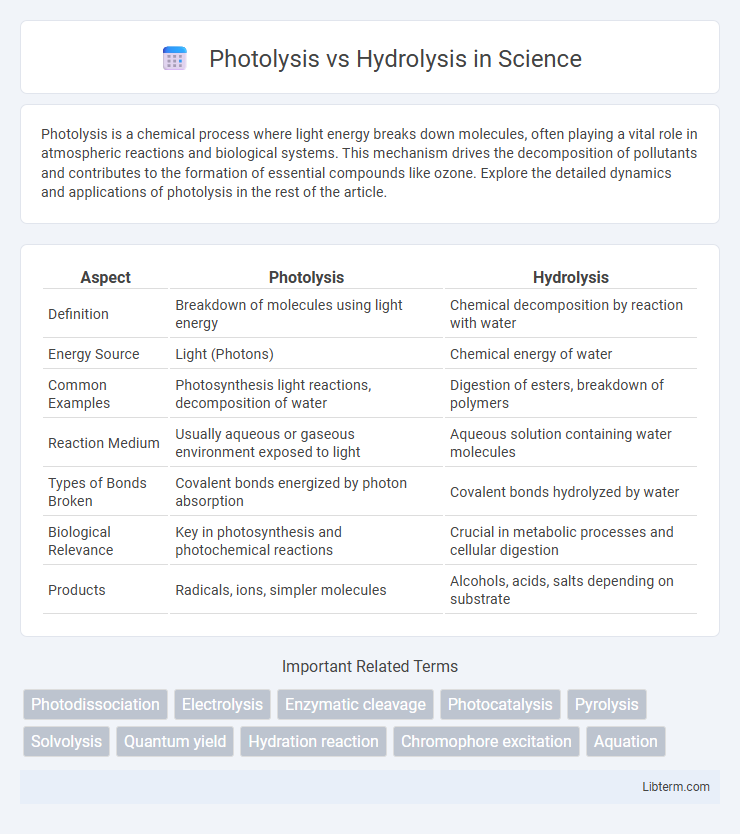
| Aspect | Photolysis | Hydrolysis |
|---|---|---|
| Definition | Breakdown of molecules using light energy | Chemical decomposition by reaction with water |
| Energy Source | Light (Photons) | Chemical energy of water |
| Common Examples | Photosynthesis light reactions, decomposition of water | Digestion of esters, breakdown of polymers |
| Reaction Medium | Usually aqueous or gaseous environment exposed to light | Aqueous solution containing water molecules |
| Types of Bonds Broken | Covalent bonds energized by photon absorption | Covalent bonds hydrolyzed by water |
| Biological Relevance | Key in photosynthesis and photochemical reactions | Crucial in metabolic processes and cellular digestion |
| Products | Radicals, ions, simpler molecules | Alcohols, acids, salts depending on substrate |
Introduction to Photolysis and Hydrolysis
Photolysis is a chemical process where light energy breaks down compounds into smaller molecules or atoms, playing a crucial role in atmospheric chemistry and biological systems such as photosynthesis. Hydrolysis involves the cleavage of chemical bonds through the reaction with water, frequently observed in biochemical reactions like the digestion of polymers into monomers. Both processes are fundamental in environmental and biological contexts, with photolysis driven by photon energy and hydrolysis by water molecules facilitating molecular transformations.
Definition of Photolysis
Photolysis is a chemical process in which molecules are broken down by photons, typically involving the absorption of light energy to cleave chemical bonds. It plays a crucial role in natural phenomena such as photosynthesis and the degradation of pollutants in atmospheric chemistry. Understanding photolysis is essential for studying energy transfer and reaction mechanisms driven by light.
Definition of Hydrolysis
Hydrolysis is a chemical process in which a molecule is cleaved into two parts through the reaction with water, commonly breaking bonds in carbohydrates, proteins, and lipids during digestion. Unlike photolysis, which involves the breakdown of molecules by photons or light energy, hydrolysis depends on the enzymatic or chemical addition of water to trigger the decomposition. This reaction is vital in various biological and chemical systems for transforming complex substrates into simpler, more reactive or absorbable components.
Key Differences Between Photolysis and Hydrolysis
Photolysis involves the decomposition of molecules by the energy of light, primarily affecting chemical bonds through photon absorption, whereas hydrolysis refers to the chemical breakdown of compounds due to reaction with water. Photolysis commonly occurs in processes like photosynthesis and water splitting during light reactions, while hydrolysis is prevalent in biological digestion and chemical synthesis where water molecules cleave bonds. The essential difference lies in the energy source driving the reaction--light energy for photolysis versus water molecules for hydrolysis--and the specific environmental conditions under which each process operates.
Mechanisms of Photolysis
Photolysis involves the breaking of chemical bonds through the absorption of light energy, often ultraviolet or visible radiation, causing molecules to dissociate into reactive radicals or ions. The mechanism typically starts with photon absorption, elevating molecules to excited electronic states that destabilize existing bonds and facilitate their cleavage. This process is fundamental in atmospheric chemistry for the degradation of pollutants and plays a crucial role in natural photosynthetic systems by initiating photochemical reactions.
Mechanisms of Hydrolysis
Hydrolysis involves the cleavage of chemical bonds through the reaction with water molecules, typically catalyzed by acids, bases, or enzymes such as hydrolases. The mechanism generally includes nucleophilic attack by a water molecule on a substrate's electrophilic center, leading to bond breaking and formation of hydroxyl and protonated groups. This process is fundamental in biological systems for the degradation of polymers like proteins, lipids, and nucleic acids, contrasting with photolysis, which uses light energy to break chemical bonds.
Applications of Photolysis
Photolysis plays a critical role in environmental science by breaking down pollutants in water and air, enhancing photocatalytic water treatment processes to remove organic contaminants and pathogens effectively. In the field of renewable energy, photolysis is fundamental in artificial photosynthesis systems, facilitating the splitting of water molecules to generate hydrogen fuel. Photolytic reactions are also utilized in photodynamic therapy for targeted cancer treatment, where light-activated compounds produce reactive oxygen species to destroy malignant cells.
Applications of Hydrolysis
Hydrolysis plays a crucial role in diverse industrial and environmental applications, such as the breakdown of complex biomolecules in wastewater treatment and the production of biofuels through the enzymatic cleavage of cellulose. It is extensively utilized in the pharmaceutical industry for drug formulation and metabolism studies, enabling the conversion of prodrugs into active compounds. Furthermore, hydrolysis facilitates food processing, including the enzymatic degradation of starches and proteins to enhance flavor, texture, and digestibility.
Environmental Impacts of Photolysis and Hydrolysis
Photolysis contributes significantly to the breakdown of pollutants by using sunlight to degrade harmful chemicals, reducing their persistence and toxicity in aquatic environments. Hydrolysis affects contaminant transformation by reacting with water molecules, influencing the degradation rates of pesticides and industrial chemicals in soil and water systems. Both processes play critical roles in natural attenuation and remediation but differ in mechanisms and environmental conditions influencing their efficiency.
Summary and Comparison Table
Photolysis involves the decomposition of compounds through the absorption of light energy, primarily ultraviolet radiation, leading to the breaking of chemical bonds without the direct involvement of water. Hydrolysis is a chemical reaction where water molecules cleave bonds in a compound, resulting in the formation of two or more products, commonly seen in biological and environmental processes. The comparison table highlights photolysis as a light-driven, non-aqueous cleavage process, while hydrolysis is a water-induced cleavage reaction often catalyzed by enzymes or acids.
Photolysis Infographic
 libterm.com
libterm.com