A molecule is the smallest unit of a chemical compound that retains its distinct properties and consists of two or more atoms bonded together. Understanding molecules is essential for exploring chemical reactions, biological processes, and material properties that influence everyday life. Dive deeper into the article to discover how molecules shape the world around you.
Table of Comparison
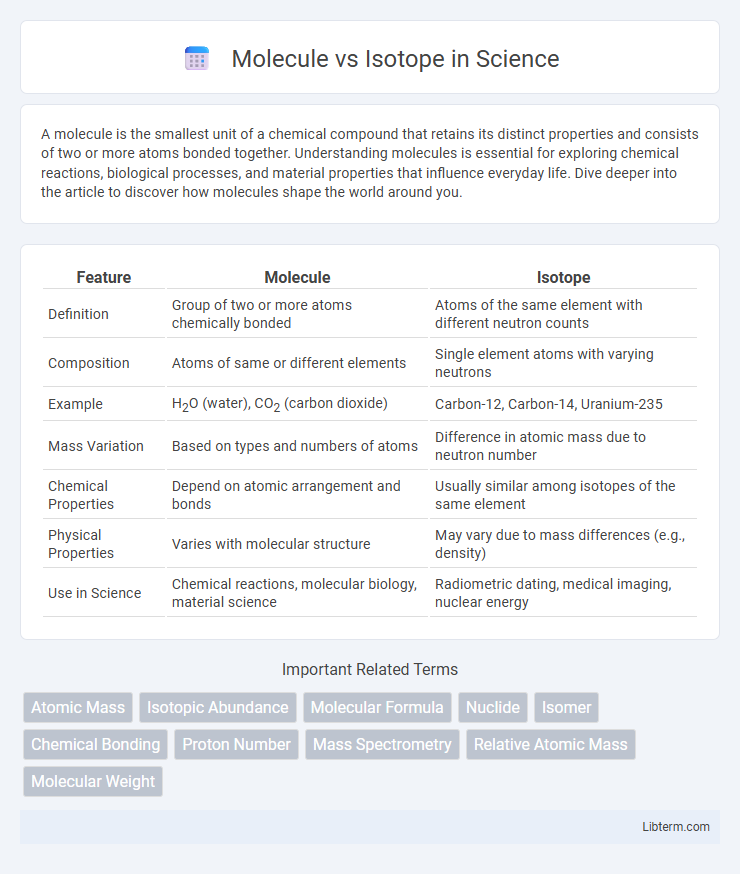
| Feature | Molecule | Isotope |
|---|---|---|
| Definition | Group of two or more atoms chemically bonded | Atoms of the same element with different neutron counts |
| Composition | Atoms of same or different elements | Single element atoms with varying neutrons |
| Example | H2O (water), CO2 (carbon dioxide) | Carbon-12, Carbon-14, Uranium-235 |
| Mass Variation | Based on types and numbers of atoms | Difference in atomic mass due to neutron number |
| Chemical Properties | Depend on atomic arrangement and bonds | Usually similar among isotopes of the same element |
| Physical Properties | Varies with molecular structure | May vary due to mass differences (e.g., density) |
| Use in Science | Chemical reactions, molecular biology, material science | Radiometric dating, medical imaging, nuclear energy |
Understanding Molecules: Definition and Structure
Molecules consist of two or more atoms chemically bonded together, forming the smallest fundamental unit of a chemical compound that retains its chemical properties. Their structure is determined by the types of atoms involved and the specific bonds, such as covalent or ionic, establishing the molecule's shape and stability. Understanding molecular geometry and bond polarity is essential for predicting reactivity and interactions in chemical and biological systems.
What Is an Isotope? Key Characteristics
An isotope is a variant of a chemical element that shares the same number of protons but differs in the number of neutrons within its atomic nucleus, resulting in distinct atomic masses. Key characteristics of isotopes include their identical chemical behavior due to the same electron configuration and their variance in nuclear stability, which can lead to radioactive decay in some isotopes. Isotopes are essential in applications such as radiometric dating, nuclear medicine, and tracing chemical pathways.
Fundamental Differences Between Molecules and Isotopes
Molecules consist of two or more atoms chemically bonded together, forming distinct chemical compounds with unique properties, while isotopes are variations of a single element differing only in neutron number within their nuclei. Molecules exhibit diverse chemical behaviors due to atom combinations, whereas isotopes share identical chemical properties but differ in atomic mass and nuclear stability. Understanding these fundamental differences is crucial for applications in chemistry, nuclear physics, and molecular biology.
The Role of Molecules in Chemical Reactions
Molecules play a crucial role in chemical reactions as they are the smallest units that retain the chemical properties of a substance and participate directly in the breaking and forming of bonds. Unlike isotopes, which are variants of elements differing in neutron number, molecules determine the specific interactions and reaction pathways through their structural configurations and bonding patterns. Understanding molecular behavior allows chemists to predict reaction outcomes, rates, and mechanisms essential for fields such as pharmaceuticals, materials science, and biochemistry.
Isotopes and Their Impact on Atomic Mass
Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons, resulting in varying atomic masses for the same element. These differences in neutron count do not affect the chemical properties significantly but have a substantial impact on the atomic mass, influencing calculations in molecular weight and atomic mass units (amu). Understanding isotopes is crucial in fields like radiometric dating, nuclear medicine, and isotope geochemistry, as they provide insights into atomic structure and stability.
Examples of Common Molecules and Their Formation
Water (H2O) and carbon dioxide (CO2) are common molecules formed through covalent bonding between hydrogen and oxygen atoms, and carbon and oxygen atoms, respectively. In contrast, isotopes are variants of a single element with differing neutron counts, such as Carbon-12 and Carbon-14, which share chemical properties but have different atomic masses. Molecular formation involves stable chemical bonds creating distinct substances, while isotopes maintain the same element identity despite mass variations.
Variations in Isotopes: Stable vs. Radioactive
Molecules consist of atoms bonded together, while isotopes are variations of a single element differing in neutron count. Stable isotopes, such as Carbon-12, do not decay over time and are commonly used in environmental and biological studies. Radioactive isotopes, like Carbon-14, emit radiation as they decay, making them valuable for dating archaeological samples and medical imaging.
Applications of Molecules in Everyday Life
Molecules, composed of two or more atoms bonded together, are fundamental to countless applications in everyday life, including the development of pharmaceuticals, the formulation of cleaning agents, and the creation of plastics. Isotopes, variants of elements with different neutron numbers, are primarily used in scientific research, medical imaging, and radiometric dating, but molecules themselves drive the chemical processes that make everyday products functional. Understanding molecular interactions enables advancements in food preservation, energy storage, and materials science, thereby enhancing the quality and convenience of daily living.
Practical Uses of Isotopes in Science and Industry
Isotopes, variants of elements with differing neutron counts, play critical roles in science and industry through applications such as medical imaging with radioactive isotopes like Technetium-99m, which enables precise diagnostic scans. In agriculture, isotopes like Nitrogen-15 facilitate soil fertility studies and fertilizer effectiveness, enhancing crop yields. Industrially, isotopes are employed in tracing processes, material testing, and radiometric dating, providing valuable insights into material composition and historical timelines.
Comparing Molecules and Isotopes: Summary Table
Molecules are composed of two or more atoms bonded chemically, forming distinct chemical substances, while isotopes are variants of a single element differing in neutron number but sharing the same atomic number. Molecules exhibit diverse chemical properties based on their atomic composition and structure, whereas isotopes of an element have nearly identical chemical behavior but vary in physical properties such as atomic mass and stability. A summary table contrasts molecules by their compound formation and varied chemical roles against isotopes' role in nuclear science, radioactive decay, and mass spectrometry applications.
Molecule Infographic
 libterm.com
libterm.com