Adiabatic processes occur without heat exchange between a system and its surroundings, ensuring energy transfer only through work. This concept is fundamental in thermodynamics, influencing the efficiency of engines and refrigeration cycles. Explore the rest of the article to understand how adiabatic principles apply to your practical applications.
Table of Comparison
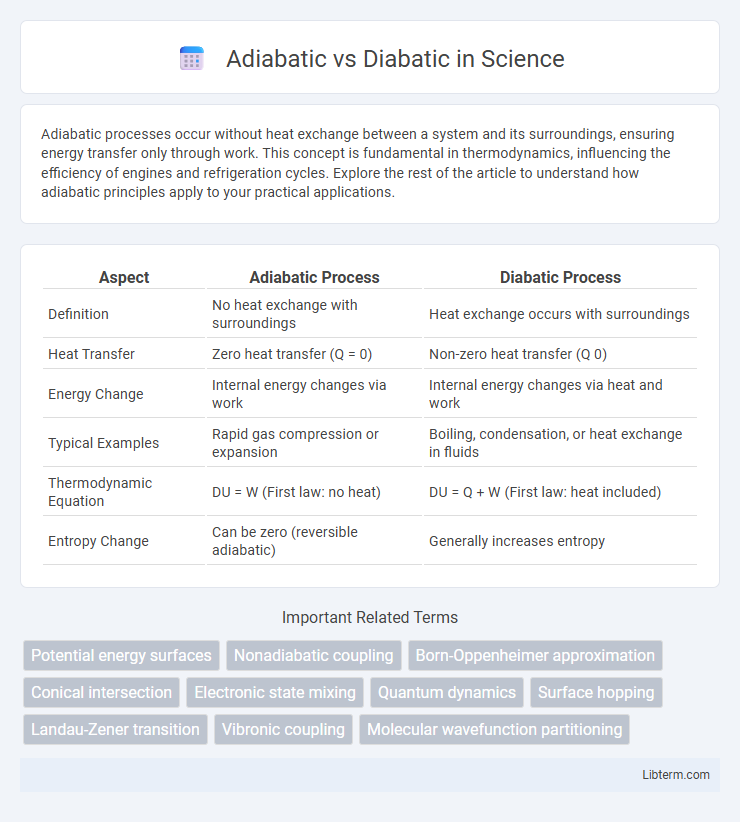
| Aspect | Adiabatic Process | Diabatic Process |
|---|---|---|
| Definition | No heat exchange with surroundings | Heat exchange occurs with surroundings |
| Heat Transfer | Zero heat transfer (Q = 0) | Non-zero heat transfer (Q 0) |
| Energy Change | Internal energy changes via work | Internal energy changes via heat and work |
| Typical Examples | Rapid gas compression or expansion | Boiling, condensation, or heat exchange in fluids |
| Thermodynamic Equation | DU = W (First law: no heat) | DU = Q + W (First law: heat included) |
| Entropy Change | Can be zero (reversible adiabatic) | Generally increases entropy |
Introduction to Adiabatic and Diabatic Processes
Adiabatic processes involve no heat exchange between a system and its surroundings, resulting in changes in pressure and temperature driven solely by internal energy variations. Diabatic processes, in contrast, allow heat transfer, causing energy changes that affect the thermodynamic state of the system through external heat addition or removal. Understanding these fundamental differences is crucial in fields like atmospheric science, thermodynamics, and chemical engineering, where energy transfer mechanisms dictate system behavior.
Fundamental Definitions
Adiabatic processes involve no heat exchange with the surroundings, ensuring that all energy changes occur through work done on or by the system, commonly observed in thermodynamics and atmospheric science. Diabatic processes entail heat transfer across system boundaries, leading to changes in internal energy through heat gain or loss, which play a critical role in weather phenomena and engineering heat exchange systems. Understanding these fundamental definitions enables accurate modeling of energy transformations in physical, chemical, and environmental contexts.
Key Differences between Adiabatic and Diabatic
Adiabatic processes occur without heat exchange between the system and surroundings, maintaining constant entropy, while diabatic processes involve heat transfer that alters the system's energy. In thermodynamics, adiabatic changes are often idealized as rapid processes preventing thermal equilibration, whereas diabatic changes are slower, allowing heat flow and temperature adjustments. The key distinction lies in the presence or absence of heat exchange, directly impacting system temperature, pressure, and entropy during state transitions.
Thermodynamic Principles Involved
Adiabatic processes involve thermodynamic changes where no heat is exchanged with the surroundings, resulting in changes in internal energy solely due to work done on or by the system. Diabatic processes allow heat transfer between the system and its environment, affecting enthalpy and entropy in accordance with the first and second laws of thermodynamics. Understanding the distinction between adiabatic and diabatic conditions is crucial for analyzing energy conservation and entropy production in thermodynamic cycles and atmospheric dynamics.
Mathematical Representation
Adiabatic processes are mathematically characterized by the condition \( Q = 0 \), leading to the differential form \( dU + PdV = 0 \), where \( U \) is internal energy, \( P \) is pressure, and \( V \) is volume. Diabatic processes involve heat exchange \( Q \neq 0 \), modifying the first law of thermodynamics to \( dU = \delta Q - PdV \), explicitly accounting for heat transfer. The governing equations for adiabatic and diabatic transformations highlight distinct energy exchange terms, crucial for thermodynamic cycle analysis and atmospheric modeling.
Real-world Examples and Applications
Adiabatic processes are crucial in meteorology, such as air parcels rising and cooling without heat exchange, driving weather patterns and cloud formation. Diabatic processes occur in climate engineering, where deliberate heat transfer through solar radiation or ocean heating impacts temperature and energy balance. Industrial applications of adiabetic compression in gas turbines improve efficiency by minimizing heat loss during rapid compression cycles.
Advantages and Limitations of Each Process
Adiabatic processes minimize heat exchange with the surroundings, making them highly efficient for insulated systems where energy loss must be prevented, yet they require ideal insulation which is often challenging to achieve in practical applications. Diabatic processes facilitate heat transfer between a system and its environment, enabling temperature control and energy exchange essential in many industrial applications, but they tend to be less energy efficient due to heat losses. The choice between adiabatic and diabatic depends on the specific process requirements for thermal management, energy efficiency, and system design constraints.
Adiabatic vs Diabatic in Quantum Systems
Adiabatic and diabatic processes describe the behavior of quantum systems under varying external conditions, particularly during transitions between energy states. In adiabatic quantum processes, the system remains in its instantaneous eigenstate due to slow parameter changes, ensuring no transitions between quantum states, which is crucial for quantum adiabatic computation and quantum annealing. Diabatic processes, in contrast, involve rapid changes causing non-adiabatic transitions where the system can jump between different energy states, impacting phenomena like Landau-Zener transitions and non-adiabatic molecular dynamics.
Common Misconceptions
Adiabatic processes are often misunderstood as requiring no heat exchange, but they specifically involve no heat transfer with the surroundings, not necessarily a temperature change within the system. Diabatic processes, in contrast, allow heat exchange, which can lead to temperature changes, debunking the misconception that they always involve external heat sources. Another common error is confusing adiabatic with isentropic processes; while adiabatic processes can be isentropic if reversible, irreversible adiabatic processes involve entropy changes.
Conclusion and Future Perspectives
Adiabatic processes maintain system isolation from heat exchange, preserving entropy, while diabatic processes involve heat transfer, leading to entropy changes and energy dissipation. Understanding the distinctions enhances modeling accuracy in thermodynamics, meteorology, and quantum mechanics, informing energy-efficient system designs. Future perspectives emphasize integrating hybrid approaches combining adiabatic and diabatic models to optimize simulations in climate modeling and quantum computing advancements.
Adiabatic Infographic
 libterm.com
libterm.com