Exothermic reactions release heat, causing the surrounding environment to increase in temperature. These reactions are common in processes like combustion and condensation, where energy is emitted as bonds form. Explore this article to understand how exothermic reactions impact your daily life and industrial applications.
Table of Comparison
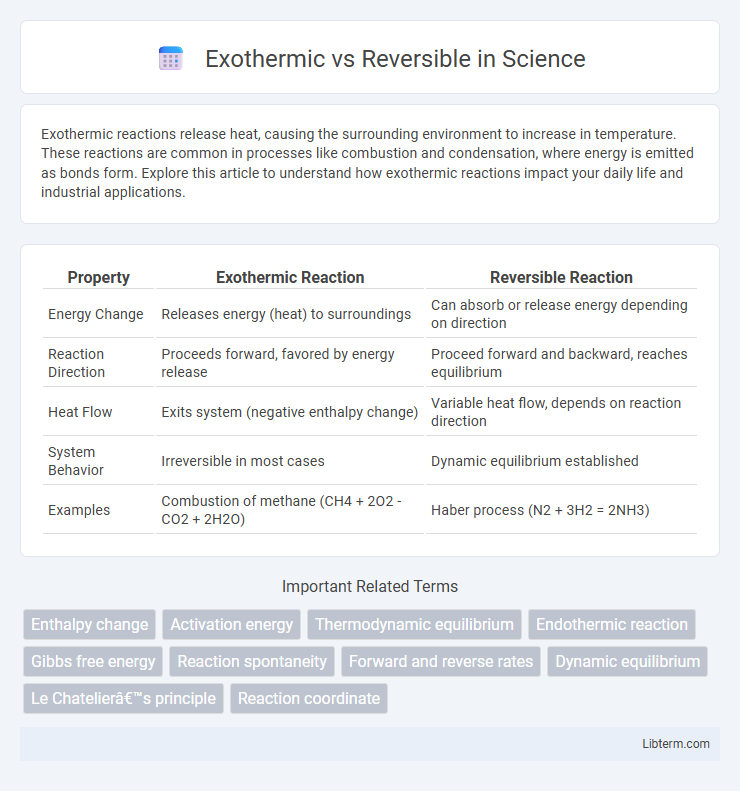
| Property | Exothermic Reaction | Reversible Reaction |
|---|---|---|
| Energy Change | Releases energy (heat) to surroundings | Can absorb or release energy depending on direction |
| Reaction Direction | Proceeds forward, favored by energy release | Proceed forward and backward, reaches equilibrium |
| Heat Flow | Exits system (negative enthalpy change) | Variable heat flow, depends on reaction direction |
| System Behavior | Irreversible in most cases | Dynamic equilibrium established |
| Examples | Combustion of methane (CH4 + 2O2 - CO2 + 2H2O) | Haber process (N2 + 3H2 = 2NH3) |
Understanding Exothermic Reactions
Exothermic reactions release energy, typically in the form of heat, as they convert reactants into products, leading to a temperature increase in the surroundings. These reactions are characterized by a negative enthalpy change (DH < 0), indicating that the energy of the products is lower than that of the reactants. Understanding the energy transfer in exothermic processes is crucial for controlling reaction conditions and optimizing industrial chemical production.
What Are Reversible Reactions?
Reversible reactions are chemical processes that can proceed in both forward and backward directions, allowing reactants to form products and products to revert to reactants under certain conditions. These reactions reach a state of dynamic equilibrium where the rates of the forward and reverse reactions are equal, resulting in constant concentrations of all species involved. Unlike exothermic reactions that release heat, reversible reactions may either absorb or release energy depending on the direction of the reaction at equilibrium.
Key Differences Between Exothermic and Reversible Reactions
Exothermic reactions release energy in the form of heat, leading to a temperature increase in the surroundings, while reversible reactions can proceed in both forward and backward directions, achieving a dynamic equilibrium. Exothermic reactions are typically spontaneous and result in the formation of stable products, whereas reversible reactions involve continuous interconversion between reactants and products without complete consumption. The key difference lies in energy flow and reaction directionality: exothermic reactions emit energy unidirectionally, whereas reversible reactions maintain an energy balance with no net energy change at equilibrium.
Energy Changes in Exothermic Processes
Exothermic processes release energy to the surroundings, resulting in a temperature increase due to the net release of heat during the reaction. These energy changes occur because the bonds formed in the products have lower potential energy than the bonds broken in the reactants. Understanding these enthalpy changes is essential for distinguishing exothermic reactions from reversible reactions where energy can be both absorbed and released depending on the direction of the reaction.
Energy Dynamics in Reversible Reactions
Energy dynamics in reversible reactions involve continuous energy exchange where the forward reaction releases energy (exothermic) while the reverse reaction absorbs energy (endothermic). This equilibrium allows the system to balance heat flow, maintaining a stable temperature and reaction rate. Understanding this thermodynamic interplay is crucial for optimizing industrial chemical processes and maximizing energy efficiency.
Examples of Exothermic Reactions
Combustion of natural gas, such as methane burning in oxygen, is a classic exothermic reaction that releases significant heat energy. The neutralization reaction between hydrochloric acid and sodium hydroxide produces water and releases heat. Respiration in living organisms, where glucose reacts with oxygen to release energy, is another vital example of an exothermic process.
Examples of Reversible Reactions
Reversible reactions frequently occur in chemical systems where the products can revert to the original reactants, such as the synthesis and dissociation of ammonia in the Haber process (N2 + 3H2 = 2NH3) and the equilibrium between carbon dioxide and water forming carbonic acid (CO2 + H2O = H2CO3). Another prominent example is the esterification reaction between carboxylic acids and alcohols, producing esters and water, which can reverse under appropriate conditions. These reactions reach a dynamic equilibrium where the forward and reverse reaction rates are equal, distinguishing them from exothermic processes that proceed predominantly in one direction releasing heat.
Practical Applications of Exothermic Reactions
Exothermic reactions find widespread practical applications in industries such as energy production, where combustion processes release heat to generate electricity, and in manufacturing, where heat generated during chemical synthesis accelerates reactions and improves efficiency. These reactions are crucial in everyday technologies like hand warmers and self-heating cans, which rely on rapid heat release to provide immediate warmth. The controlled release of heat in exothermic reactions also underpins safety mechanisms in fire suppression systems and industrial cooling processes.
Industrial Uses of Reversible Reactions
Reversible reactions play a crucial role in industrial processes such as ammonia synthesis in the Haber-Bosch process, where equilibrium manipulation enhances yield and energy efficiency. These reactions enable dynamic control over product formation by adjusting conditions like temperature and pressure, optimizing resource utilization and reducing waste. Industries exploit reversible systems to recover valuable reactants and minimize operational costs, contributing to sustainable manufacturing practices.
Exothermic vs Reversible: Which Is More Efficient?
Exothermic reactions release energy in the form of heat, making them highly efficient for processes requiring immediate energy output, such as combustion or cellular respiration. Reversible reactions, however, allow the system to reach equilibrium, efficiently conserving energy by minimizing losses in processes like enzyme catalysis and metabolic pathways. The efficiency depends on the application: exothermic reactions excel in rapid energy release, while reversible reactions optimize energy utilization by balancing forward and backward reaction rates.
Exothermic Infographic
 libterm.com
libterm.com