Amphipathic molecules possess both hydrophilic (water-attracting) and hydrophobic (water-repelling) regions, enabling them to interact with diverse environments. This unique structure is crucial for forming biological membranes and facilitating molecular transport. Discover how amphipathic properties impact your understanding of cell function and biochemistry in the rest of this article.
Table of Comparison
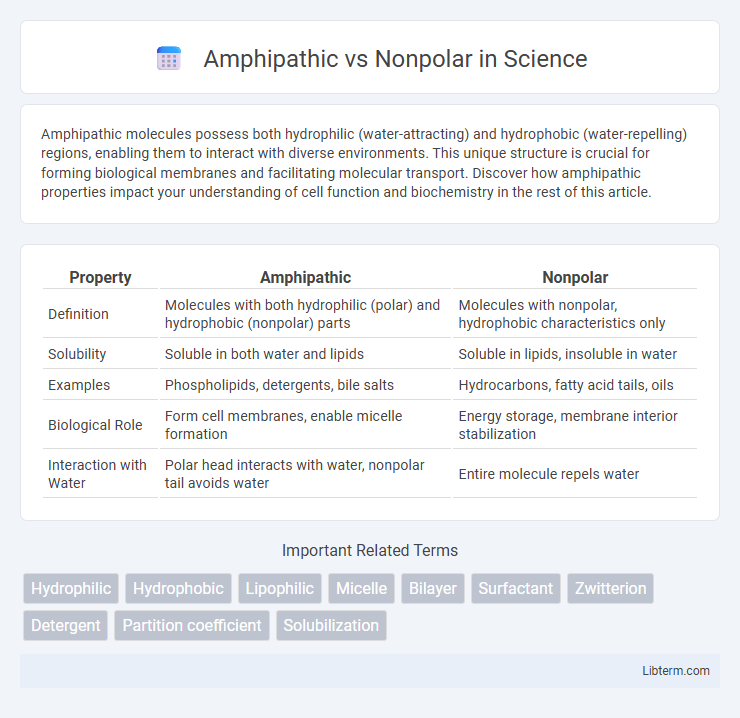
| Property | Amphipathic | Nonpolar |
|---|---|---|
| Definition | Molecules with both hydrophilic (polar) and hydrophobic (nonpolar) parts | Molecules with nonpolar, hydrophobic characteristics only |
| Solubility | Soluble in both water and lipids | Soluble in lipids, insoluble in water |
| Examples | Phospholipids, detergents, bile salts | Hydrocarbons, fatty acid tails, oils |
| Biological Role | Form cell membranes, enable micelle formation | Energy storage, membrane interior stabilization |
| Interaction with Water | Polar head interacts with water, nonpolar tail avoids water | Entire molecule repels water |
Introduction to Molecular Polarity
Molecular polarity arises from the distribution of electrical charges across a molecule, influencing interactions with solvents and other molecules. Amphipathic molecules contain both polar (hydrophilic) and nonpolar (hydrophobic) regions, enabling them to interact with diverse environments, such as forming micelles in aqueous solutions. Nonpolar molecules lack charged regions, exhibiting symmetric electron distribution that limits their solubility to nonpolar solvents.
Defining Amphipathic Molecules
Amphipathic molecules contain both hydrophilic (water-attracting) and hydrophobic (water-repelling) regions, allowing them to interact with polar and nonpolar environments simultaneously. In contrast, nonpolar molecules consist entirely of hydrophobic components, lacking affinity for aqueous solutions. This dual nature of amphipathic molecules is critical in biological systems, particularly in the formation of cell membranes where phospholipids arrange themselves into bilayers.
Understanding Nonpolar Molecules
Nonpolar molecules consist primarily of carbon and hydrogen atoms with symmetrical electron distribution, resulting in no significant charge difference across the molecule. Unlike amphipathic molecules, which contain both hydrophilic and hydrophobic regions, nonpolar molecules lack polarity and do not interact favorably with water, making them hydrophobic. Their nonpolar nature is critical in biological systems for forming lipid bilayers and influencing membrane fluidity.
Key Structural Differences
Amphipathic molecules contain both hydrophilic (polar) and hydrophobic (nonpolar) regions, typically featuring a distinct polar head group and one or more nonpolar hydrocarbon tails. Nonpolar molecules consist entirely of hydrophobic hydrocarbon chains or rings, lacking any significant polarity or charge. This structural difference enables amphipathic molecules to interact with both aqueous and lipid environments, crucial for forming biological membranes, whereas nonpolar molecules primarily interact through van der Waals forces in nonpolar solvents.
Polarity and Solubility
Amphipathic molecules contain both polar and nonpolar regions, allowing them to interact with diverse solvents by aligning their polar segments with water and nonpolar segments with lipids, enhancing solubility in both aqueous and lipid environments. Nonpolar molecules lack significant polarity, resulting in poor solubility in water but high solubility in nonpolar solvents due to their hydrophobic nature. The distinct polarity of amphipathic compounds enables crucial biological functions such as membrane formation and molecular transport, while nonpolar molecules primarily participate in hydrophobic interactions.
Biological Roles of Amphipathic Molecules
Amphipathic molecules contain both hydrophilic and hydrophobic regions, enabling them to form biological membranes by self-assembling into bilayers that compartmentalize cells and organelles. These molecules, such as phospholipids, are crucial for membrane fluidity, selective permeability, and cell signaling processes. In contrast, nonpolar molecules primarily serve as energy storage or structural components without the ability to form stable membranes due to their uniform hydrophobic nature.
Functions of Nonpolar Compounds in Biology
Nonpolar compounds primarily function as essential components of biological membranes, providing structural integrity and creating hydrophobic barriers that regulate the passage of molecules. These compounds serve as energy reserves, exemplified by lipids such as triglycerides that store and release energy efficiently. Nonpolar molecules also participate in cell signaling and insulation, aiding in maintaining cellular communication and thermal regulation.
Amphipathic vs Nonpolar: Interactions with Water
Amphipathic molecules possess both hydrophilic (water-attracting) and hydrophobic (water-repelling) regions, allowing them to interact effectively with water by forming structures like micelles or bilayers that stabilize their interaction with aqueous environments. Nonpolar molecules lack charged or polar groups, resulting in minimal interaction with water and causing them to aggregate or separate rather than dissolve, driven by hydrophobic effects. These contrasting behaviors influence biological membrane formation, protein folding, and the solubility of various substances in water-based systems.
Applications in Industry and Medicine
Amphipathic molecules, possessing both hydrophilic and hydrophobic regions, are essential in pharmaceutical formulations for drug delivery systems, enabling targeted release and improved solubility of hydrophobic drugs. Nonpolar compounds, characterized by their hydrophobic nature, are widely used in industrial applications such as lubricants, coatings, and solvents where water resistance and low polarity are crucial. The contrasting properties of amphipathic and nonpolar molecules allow tailored design of emulsifiers, surfactants, and membrane-mimetic materials in biomedical engineering and industrial manufacturing.
Summary: Comparing Amphipathic and Nonpolar Properties
Amphipathic molecules contain both hydrophilic and hydrophobic regions, allowing them to interact with polar and nonpolar environments, essential for forming biological membranes and micelles. Nonpolar molecules lack charged or polar groups, resulting in hydrophobic behavior and poor solubility in water, making them primarily soluble in nonpolar solvents. The contrasting properties of amphipathic and nonpolar molecules dictate their distinct roles in cellular processes and chemical interactions.
Amphipathic Infographic
 libterm.com
libterm.com