Charged particles play a crucial role in various physical and chemical processes, influencing everything from electrical conductivity to chemical reactions. Understanding how these particles interact can provide insights into phenomena like electromagnetic fields and plasma behavior. Explore the full article to discover how charged entities impact your everyday life and modern technology.
Table of Comparison
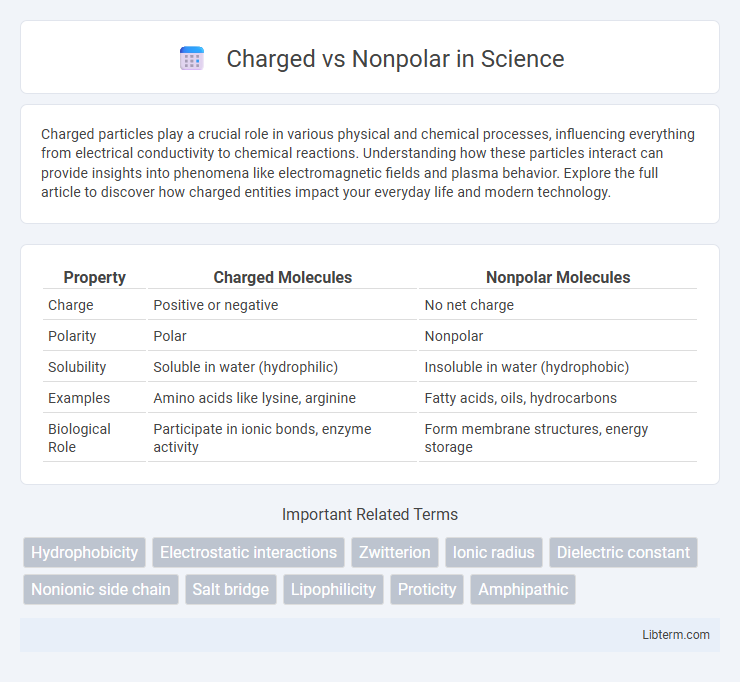
| Property | Charged Molecules | Nonpolar Molecules |
|---|---|---|
| Charge | Positive or negative | No net charge |
| Polarity | Polar | Nonpolar |
| Solubility | Soluble in water (hydrophilic) | Insoluble in water (hydrophobic) |
| Examples | Amino acids like lysine, arginine | Fatty acids, oils, hydrocarbons |
| Biological Role | Participate in ionic bonds, enzyme activity | Form membrane structures, energy storage |
Introduction to Charged and Nonpolar Molecules
Charged molecules, also known as ions, possess an electrical charge due to the loss or gain of electrons, making them hydrophilic and highly reactive in biological systems. Nonpolar molecules lack a significant charge difference between atoms, resulting in a neutral charge that promotes hydrophobic interactions and influences molecular behavior in lipid membranes and organic solvents. Understanding the distinction between charged and nonpolar molecules is essential for studying molecular interactions, solubility, and cellular functions.
Chemical Structure Differences
Charged molecules contain functional groups like carboxyl (-COO-) or amino (-NH3+) groups that ionize and carry positive or negative charges, influencing their solubility and reactivity. Nonpolar molecules primarily consist of hydrocarbon chains or rings lacking significant electronegative atoms, resulting in an even distribution of electrons and no net charge. These chemical structure differences affect molecular interactions, with charged molecules engaging in ionic and hydrogen bonding, while nonpolar molecules rely on van der Waals forces.
Types of Charged Molecules
Charged molecules, also known as ions, include cations with a positive charge and anions with a negative charge, which arise from the loss or gain of electrons, respectively. Examples of charged molecules include amino acids with ionizable side chains such as lysine (positively charged) and glutamate (negatively charged), key for protein function and interaction. These charged species contrast with nonpolar molecules, which lack significant charge separation and are typically hydrophobic, influencing molecular behavior and solubility in biological systems.
Characteristics of Nonpolar Molecules
Nonpolar molecules exhibit an even distribution of electrical charge, resulting in no permanent dipole moment and low polarity. These molecules are typically composed of atoms with similar electronegativities, leading to symmetric charge distribution and poor solubility in polar solvents like water. Common examples include hydrocarbons such as methane and oils, which demonstrate hydrophobic behavior due to their nonpolar characteristics.
Solubility: Charged vs Nonpolar
Charged molecules, such as ions and polar compounds, exhibit high solubility in water due to their ability to form strong electrostatic and hydrogen bonds with water molecules. Nonpolar molecules lack these charges and tend to be hydrophobic, resulting in low solubility in water but higher solubility in nonpolar solvents like hexane or benzene. The solubility difference is governed by the principle "like dissolves like," where polar solvents dissolve charged or polar solutes more effectively than nonpolar solutes.
Interactions with Water
Charged molecules, such as ions and polar compounds, exhibit strong interactions with water due to their ability to form hydrogen bonds and electrostatic attractions, enhancing their solubility. Nonpolar molecules lack these charges and have weak Van der Waals interactions with water, leading to poor solubility and the tendency to aggregate to minimize exposure to the aqueous environment. These distinct behaviors drive crucial biological processes like protein folding and membrane formation by influencing molecular interactions within cells.
Biological Roles and Functions
Charged amino acids, such as lysine and glutamate, play critical roles in enzyme active sites and membrane transport by facilitating electrostatic interactions and proton transfer essential for biochemical reactions. Nonpolar amino acids, including leucine and valine, contribute to protein stability and membrane integration through hydrophobic interactions that maintain structural integrity and proper folding. The distinct chemical properties of charged and nonpolar residues enable diverse biological functions, influencing protein dynamics, signaling pathways, and cellular compartmentalization.
Common Examples in Nature
Charged molecules like amino acids arginine and glutamate play essential roles in protein structure and function due to their polarity and ability to form ionic bonds. Nonpolar molecules such as methane and the side chains of amino acids like leucine and valine are typically found in hydrophobic regions of proteins and lipid membranes, contributing to structural stability through hydrophobic interactions. These chemical properties drive vital biological processes like enzyme catalysis and membrane formation.
Implications in Chemical Reactions
Charged molecules, such as ions, exhibit strong electrostatic interactions that significantly influence reaction rates and mechanisms by stabilizing transition states or intermediates. Nonpolar molecules primarily engage through London dispersion forces and hydrophobic interactions, often resulting in slower reaction kinetics and distinct selectivity profiles in solvents. These differences impact catalyst design, solubility, and the formation of reaction complexes in organic and inorganic chemistry.
Summary: Key Differences
Charged molecules possess an electrical charge due to the presence of ions, making them hydrophilic and highly reactive in aqueous environments. Nonpolar molecules lack significant charge distribution, resulting in hydrophobic characteristics and limited solubility in water. The key differences revolve around their polarity, solubility, and interaction with other substances in biological and chemical systems.
Charged Infographic
 libterm.com
libterm.com