Radical ideas have the power to challenge conventional thinking and inspire profound change in society. Embracing radical perspectives can foster innovation and open new pathways for progress in various fields. Discover how radical approaches can transform your outlook and influence the world by exploring the rest of this article.
Table of Comparison
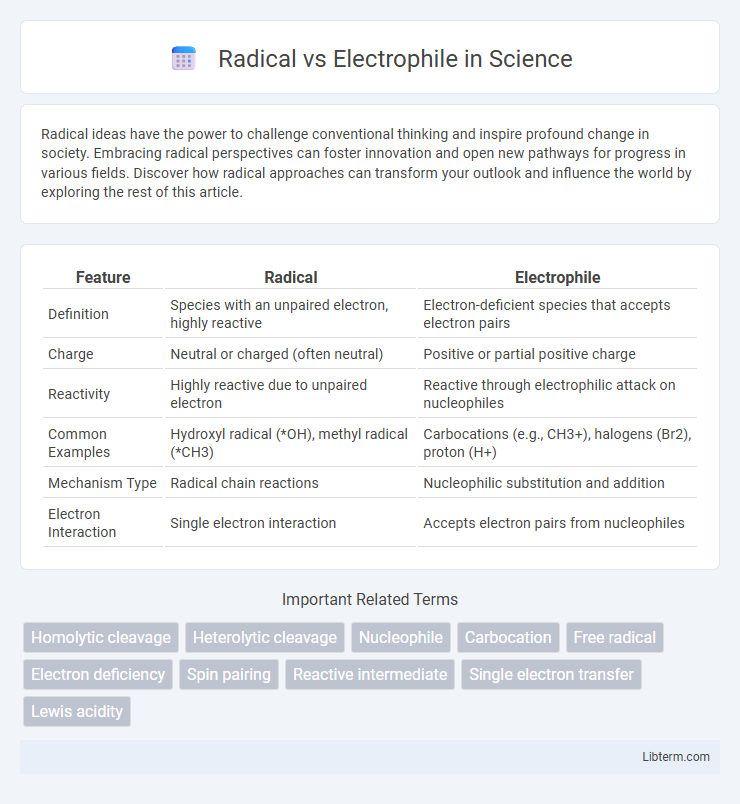
| Feature | Radical | Electrophile |
|---|---|---|
| Definition | Species with an unpaired electron, highly reactive | Electron-deficient species that accepts electron pairs |
| Charge | Neutral or charged (often neutral) | Positive or partial positive charge |
| Reactivity | Highly reactive due to unpaired electron | Reactive through electrophilic attack on nucleophiles |
| Common Examples | Hydroxyl radical (*OH), methyl radical (*CH3) | Carbocations (e.g., CH3+), halogens (Br2), proton (H+) |
| Mechanism Type | Radical chain reactions | Nucleophilic substitution and addition |
| Electron Interaction | Single electron interaction | Accepts electron pairs from nucleophiles |
Introduction to Radicals and Electrophiles
Radicals are species containing unpaired electrons that exhibit high reactivity in organic reactions, often generated via homolytic bond cleavage. Electrophiles are electron-deficient molecules or ions that seek electron-rich sites to form covalent bonds, typically involved in polar reactions through heterolytic bond cleavage. Understanding the distinct reactive behaviors of radicals and electrophiles is essential for predicting mechanisms in organic synthesis and reaction pathways.
Defining Radicals: Structure and Formation
Radicals are species containing an unpaired electron in their outer shell, often symbolized by a dot, resulting in high reactivity due to electron deficiency. Structurally, radicals can be carbon-centered, oxygen-centered, or halogen-centered, formed through homolytic bond cleavage where each atom retains one electron from the broken bond. Formation mechanisms include photolysis, thermal decomposition, and redox reactions, all essential processes in organic synthesis and polymerization reactions.
What Are Electrophiles? Key Characteristics
Electrophiles are chemical species that seek electrons due to their electron-deficient nature, making them positively charged or neutral molecules with vacant orbitals. Key characteristics of electrophiles include their ability to accept electron pairs from nucleophiles during chemical reactions, high reactivity toward electron-rich sites, and frequent presence of partial positive charges or Lewis acid behavior. Examples of electrophiles include carbocations, halogens, and molecules like sulfur trioxide (SO3) that participate in electrophilic addition or substitution reactions.
Electronic Structure Comparison: Radicals vs. Electrophiles
Radicals contain unpaired electrons in their molecular orbitals, making them highly reactive due to electron deficiency localized in one orbital, while electrophiles typically possess vacant orbitals or partially positive centers that allow them to accept electron pairs during chemical reactions. The electronic structure of radicals exhibits one singly occupied molecular orbital (SOMO), whereas electrophiles often feature empty low-energy orbitals such as the LUMO that facilitate nucleophilic attack. This difference in electron configuration governs their distinct reactivities: radicals engage in homolytic bond cleavage and single-electron transfers, whereas electrophiles proceed with heterolytic bond formation by accepting electron pairs.
Generation Mechanisms of Radicals and Electrophiles
Radicals are generated primarily through homolytic bond cleavage, which involves the equal splitting of a covalent bond to form two species each with an unpaired electron, commonly initiated by heat, light, or radical initiators like peroxides. Electrophiles arise via heterolytic bond cleavage or by the presence of electron-deficient atoms or species, often formed from protonation, metal ion coordination, or the loss of a leaving group, resulting in a positive charge or partial positive character. The distinct generation mechanisms influence their reactivity profiles, where radicals are neutral and highly reactive due to unpaired electrons, while electrophiles seek electron-rich sites because of their electron deficiency.
Reaction Pathways and Mechanisms
Radical reaction pathways typically involve homolytic bond cleavage, generating species with unpaired electrons that undergo propagation steps such as abstraction or addition, favoring chain reactions with high reactivity and selectivity. Electrophile reaction mechanisms proceed via heterolytic bond cleavage, forming positively charged intermediates that interact with nucleophiles through concerted or stepwise processes like nucleophilic substitution or electrophilic addition. Understanding the distinction between radical and electrophile mechanisms is critical for predicting product distribution and optimizing conditions in organic synthesis.
Role in Organic Synthesis
Radicals initiate chain reactions in organic synthesis by generating highly reactive intermediates that enable transformations such as halogenation and polymerization. Electrophiles act as electron-pair acceptors, facilitating nucleophilic substitutions and additions crucial for constructing complex molecules. Both species drive distinct mechanistic pathways that expand synthetic versatility and selectivity in forming carbon-carbon and carbon-heteroatom bonds.
Stability and Reactivity Differences
Radicals exhibit moderate stability due to an unpaired electron, often stabilized by resonance or hyperconjugation, while electrophiles tend to be more reactive, seeking electron-rich species to achieve a stable octet. The stability of radicals depends on the delocalization of the unpaired electron, whereas electrophiles' reactivity is influenced by their positive charge or electron-deficient sites. Electrophiles typically react faster than radicals in polar reactions, while radicals participate predominantly in chain reactions through homolytic bond cleavage.
Common Examples and Real-World Applications
Radicals such as hydroxyl (*OH) and alkyl radicals play crucial roles in polymerization processes and combustion reactions, while electrophiles like carbocations and acylium ions are key intermediates in organic synthesis and pharmaceutical drug development. Radical initiators like benzoyl peroxide enable controlled radical polymerization for manufacturing plastics, whereas electrophiles participate in electrophilic aromatic substitution reactions critical for producing dyes and agrochemicals. Understanding the distinct reactivities of radicals and electrophiles facilitates advancements in industrial chemistry and material science applications.
Summary: Choosing Between Radical and Electrophilic Pathways
Radical pathways involve uncharged species with unpaired electrons, favoring reactions under conditions that stabilize radicals, such as thermal or photochemical initiation. Electrophilic pathways feature positively charged or electron-deficient species that seek electron-rich sites, typically proceeding through polar mechanisms influenced by substrate nucleophilicity and solvent polarity. Selecting between radical and electrophilic mechanisms depends on substrate structure, reaction conditions, and the presence of radical initiators or electrophilic catalysts to optimize yield and selectivity.
Radical Infographic
 libterm.com
libterm.com