Mitogenic substances stimulate cell division and proliferation by activating specific signaling pathways that promote DNA synthesis and mitosis. Understanding mitogenic mechanisms is crucial for advancements in cancer research, tissue regeneration, and developmental biology. Discover how mitogenic factors influence cellular growth and explore their potential applications in the full article.
Table of Comparison
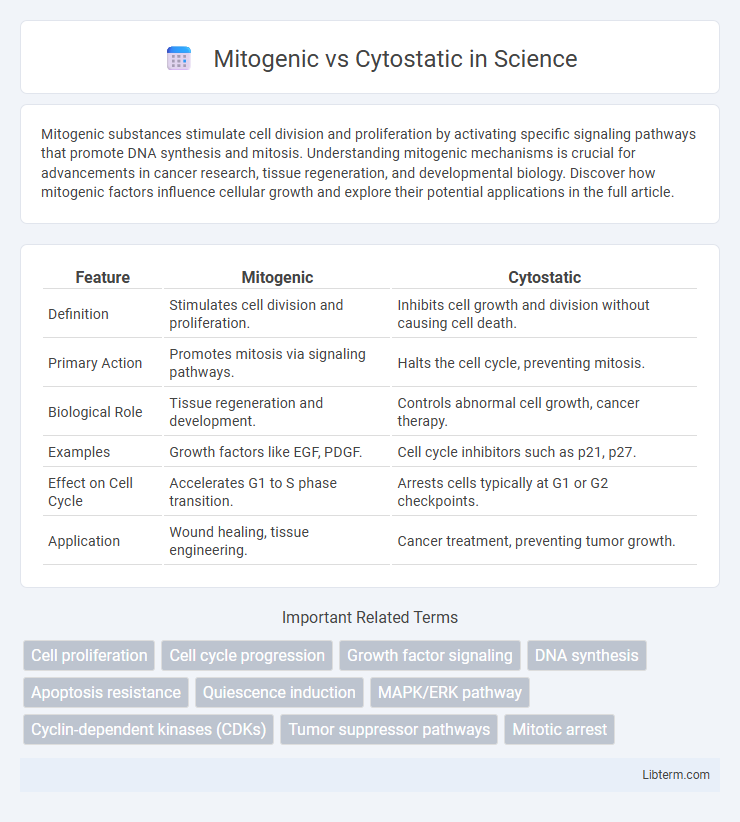
| Feature | Mitogenic | Cytostatic |
|---|---|---|
| Definition | Stimulates cell division and proliferation. | Inhibits cell growth and division without causing cell death. |
| Primary Action | Promotes mitosis via signaling pathways. | Halts the cell cycle, preventing mitosis. |
| Biological Role | Tissue regeneration and development. | Controls abnormal cell growth, cancer therapy. |
| Examples | Growth factors like EGF, PDGF. | Cell cycle inhibitors such as p21, p27. |
| Effect on Cell Cycle | Accelerates G1 to S phase transition. | Arrests cells typically at G1 or G2 checkpoints. |
| Application | Wound healing, tissue engineering. | Cancer treatment, preventing tumor growth. |
Understanding Mitogenic and Cytostatic Concepts
Mitogenic agents stimulate cell division and proliferation by activating signaling pathways that promote cell cycle progression. Cytostatic agents inhibit cell growth and proliferation by inducing cell cycle arrest without causing cell death. Understanding the molecular mechanisms of mitogenic and cytostatic responses is crucial for developing targeted therapies in cancer and regenerative medicine.
Defining Mitogenic Activity
Mitogenic activity refers to the process by which certain stimuli, such as growth factors or mitogens, trigger cellular division and proliferation by promoting the G1 phase progression in the cell cycle. This activity contrasts with cytostatic effects, which inhibit cell growth and division without causing cell death. Understanding mitogenic signals is crucial for research in cancer biology and tissue regeneration, as these pathways regulate cell population expansion.
Exploring Cytostatic Mechanisms
Cytostatic mechanisms primarily involve the inhibition of cell cycle progression, preventing mitogenic signals from driving cell proliferation. These mechanisms regulate key checkpoints by activating proteins such as p21 and p27, which inhibit cyclin-dependent kinases (CDKs) and halt the transition from G1 to S phase. Understanding cytostatic pathways provides crucial insights into controlling uncontrolled cell growth in diseases like cancer.
Key Molecular Pathways in Mitogenesis
Mitogenic signaling primarily involves key molecular pathways such as the MAPK/ERK cascade, PI3K/Akt pathway, and JAK/STAT signaling, which stimulate cell cycle progression and promote cellular proliferation through activation of cyclin-dependent kinases and transcription factors like c-Myc and ELK1. In contrast, cytostatic mechanisms often trigger cell cycle arrest via upregulation of inhibitors like p21 and p27, mediated by pathways such as p53 and TGF-b signaling that inhibit mitogenic signals. Understanding the interplay between mitogenic pathways and cytostatic checkpoints is critical for targeting proliferative diseases, including cancer.
Cytostatic Pathways and Cellular Regulation
Cytostatic pathways regulate cell proliferation by arresting the cell cycle without inducing cell death, primarily through mechanisms involving cyclin-dependent kinase inhibitors such as p21 and p27. These pathways maintain cellular homeostasis by modulating signaling cascades like the TGF-b pathway and p53-mediated responses, which enforce checkpoint controls and prevent uncontrolled mitogenic stimulation. Cytostatic regulation is crucial in cancer biology for limiting tumor growth and promoting cellular senescence, contrasting with mitogenic signals that actively drive cell division.
Differences Between Mitogenic and Cytostatic Responses
Mitogenic responses trigger cell division by activating signaling pathways that promote progression through the cell cycle, primarily involving growth factors and mitogens. Cytostatic responses, in contrast, inhibit cell proliferation by inducing cell cycle arrest, often through mechanisms like tumor suppressor activation or cellular stress signals. The key difference lies in mitogenic signals driving cell growth and replication, whereas cytostatic signals prevent unchecked cell division to maintain tissue homeostasis and prevent tumorigenesis.
Biological Implications in Health and Disease
Mitogenic signals stimulate cell division by activating pathways that promote cell cycle progression, playing a crucial role in tissue growth, repair, and oncogenesis. Cytostatic factors induce cell cycle arrest, maintaining cellular homeostasis and preventing uncontrolled proliferation, which is essential for tumor suppression and managing chronic diseases. Dysregulation of mitogenic and cytostatic balance contributes to pathological conditions such as cancer, fibrosis, and degenerative disorders by disrupting normal cellular growth and differentiation processes.
Mitogenic vs Cytostatic Effects in Cancer Therapy
Mitogenic effects in cancer therapy stimulate cell division and proliferation, often contributing to tumor growth, while cytostatic effects inhibit cell cycle progression, preventing cancer cell proliferation without inducing cell death. Therapies with cytostatic properties aim to halt tumor expansion by arresting cells in specific phases of the cell cycle, thereby limiting cancer progression. Understanding the balance between mitogenic and cytostatic responses is crucial for optimizing targeted treatments and improving therapeutic outcomes in oncology.
Experimental Approaches to Study Mitogenic and Cytostatic Actions
Experimental approaches to study mitogenic and cytostatic actions often utilize cell proliferation assays like BrdU incorporation or Ki-67 staining to quantify mitogenic activity. Flow cytometry analysis of cell cycle phases provides insights into cytostatic effects by revealing cell cycle arrest at specific checkpoints. Time-lapse microscopy and molecular markers such as cyclins, CDKs, and p21 also help differentiate between stimulation of cell division and inhibition of cell growth.
Future Directions in Therapeutic Applications
Future directions in therapeutic applications are increasingly exploring mitogenic agents to stimulate controlled cell growth for tissue regeneration and repair, particularly in neurodegenerative diseases and wound healing. Cytostatic treatments are advancing with targeted therapies that inhibit cancer cell proliferation by modulating cell cycle checkpoints and signaling pathways such as CDK4/6 inhibitors in breast cancer. Integration of precision medicine with molecular profiling is driving development of combination therapies that balance mitogenic stimulation with cytostatic control to enhance treatment efficacy while minimizing adverse effects.
Mitogenic Infographic
 libterm.com
libterm.com