Redox titration is an analytical technique used to determine the concentration of an unknown oxidizing or reducing agent through a redox reaction. This method involves the gradual addition of a titrant until the reaction reaches its equivalence point, often indicated by a color change using an appropriate indicator. Discover the detailed process and applications of redox titration in the rest of the article.
Table of Comparison
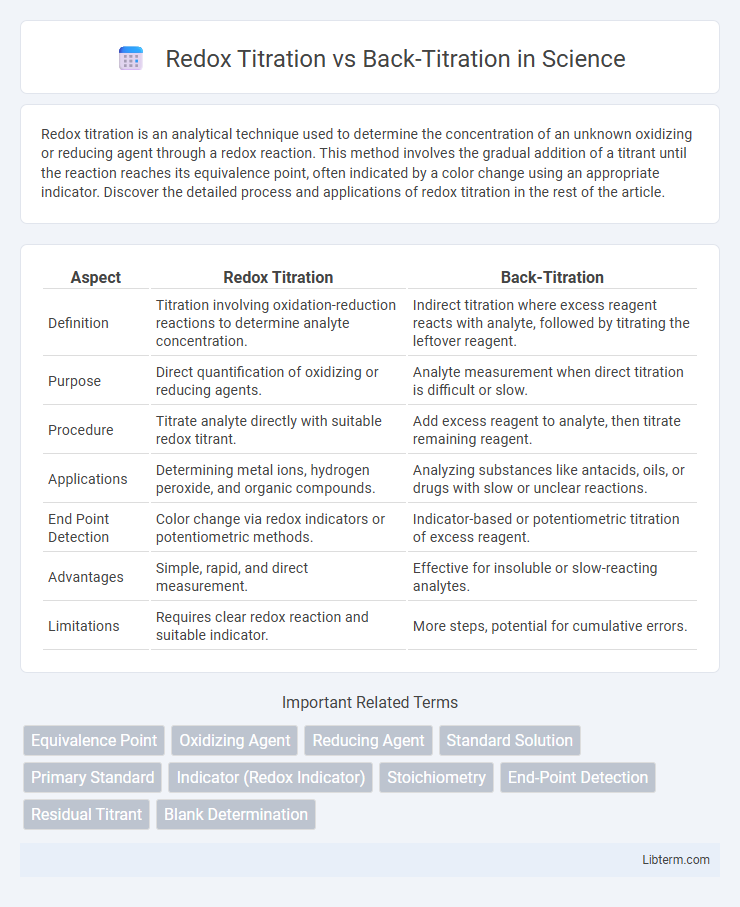
| Aspect | Redox Titration | Back-Titration |
|---|---|---|
| Definition | Titration involving oxidation-reduction reactions to determine analyte concentration. | Indirect titration where excess reagent reacts with analyte, followed by titrating the leftover reagent. |
| Purpose | Direct quantification of oxidizing or reducing agents. | Analyte measurement when direct titration is difficult or slow. |
| Procedure | Titrate analyte directly with suitable redox titrant. | Add excess reagent to analyte, then titrate remaining reagent. |
| Applications | Determining metal ions, hydrogen peroxide, and organic compounds. | Analyzing substances like antacids, oils, or drugs with slow or unclear reactions. |
| End Point Detection | Color change via redox indicators or potentiometric methods. | Indicator-based or potentiometric titration of excess reagent. |
| Advantages | Simple, rapid, and direct measurement. | Effective for insoluble or slow-reacting analytes. |
| Limitations | Requires clear redox reaction and suitable indicator. | More steps, potential for cumulative errors. |
Introduction to Redox Titration and Back-Titration
Redox titration involves the quantitative determination of an analyte through oxidation-reduction reactions where electrons are transferred between reactants, commonly utilizing standard solutions such as potassium permanganate or iodine. Back-titration is an indirect analytical method used when the analyte is not suitable for direct titration, involving the addition of a known excess reagent followed by titration of the unreacted excess, enhancing accuracy for substances like calcium carbonate or pharmaceuticals. Both techniques are essential in analytical chemistry for precise concentration measurements, particularly in complex matrices or when endpoint detection is challenging.
Fundamental Principles of Redox Titration
Redox titration involves the quantitative determination of an analyte through a redox reaction between the analyte and a titrant with a known oxidizing or reducing agent, where electrons are transferred to establish the equivalence point. The fundamental principle is based on the precise measurement of the change in oxidation states of the involved species, allowing stoichiometric calculation of the analyte concentration. Indicators or potentiometric methods are employed to detect the end point, ensuring accuracy in redox reactions compared to the indirect measurement used in back-titration.
Understanding Back-Titration Techniques
Back-titration is a quantitative analytical technique used when direct titration of the analyte is difficult due to slow reaction rates or insoluble products. It involves adding an excess reagent to react with the analyte, then titrating the remaining reagent with a secondary titrant to determine the analyte concentration indirectly. This method is particularly effective in redox reactions where endpoint detection is challenging or in complex matrices requiring precise measurement.
Key Chemical Reactions in Redox Titration
Redox titration involves electron transfer reactions between the titrant and analyte, where oxidation and reduction half-reactions occur at the electrode or reaction interface, exemplified by the reaction between potassium permanganate (MnO4-) and iron(II) ions (Fe2+), where MnO4- is reduced and Fe2+ is oxidized. The equivalence point is reached when the number of electrons lost in oxidation equals the number gained in reduction, allowing precise determination of analyte concentration. In contrast, back-titration involves adding an excess reagent to the analyte and then titrating the unreacted reagent, which is particularly useful when direct redox titration is difficult due to reaction kinetics or solubility issues.
Step-by-Step Redox Titration Procedure
Redox titration involves a step-by-step procedure starting with preparing the analyte solution and selecting an appropriate redox indicator. The titrant, usually an oxidizing or reducing agent, is slowly added until the equivalence point, indicated by a color change, is reached. Accurate measurement of the volume of titrant used allows calculation of the oxidizing or reducing agent concentration in the analyte solution.
Applications of Back-Titration in Analytical Chemistry
Back-titration is widely applied in analytical chemistry to determine concentrations of substances that react slowly or have insoluble products, such as in metal ore analysis and pharmaceutical assays. It is particularly useful for analyzing calcium carbonate content in cement and soils where direct titration is impractical. This method enhances accuracy by titrating the excess reagent, making it ideal for complex matrices and weakly reactive substances.
Advantages and Limitations of Redox Titration
Redox titration offers precise determination of analytes through direct electron transfer reactions, providing high accuracy and specificity for substances that undergo oxidation or reduction. Its main advantages include rapid reaction times and minimal interference from other ions, making it suitable for quantitative analysis of metal ions and oxidizing agents. Limitations involve the requirement for suitable redox indicators and potential difficulty in endpoint detection when the color change is subtle or reactions are slow.
Comparative Analysis: Redox Titration vs Back-Titration
Redox titration involves the direct measurement of the analyte's oxidation or reduction by using a suitable redox reagent, allowing precise determination of the analyte's concentration through electron transfer reactions. Back-titration, in contrast, is an indirect method where an excess reagent is added to the analyte and the remaining excess is titrated, making it ideal for reactions with slow kinetics or insoluble products. The comparative advantage of redox titration lies in its straightforward procedure and accuracy, whereas back-titration offers versatility and applicability in cases where direct titration is challenging or impractical.
Common Errors and Troubleshooting Tips
Redox titration and back-titration often face common errors such as inaccurate endpoint detection due to color change ambiguities or incomplete reactions. Ensuring proper standardization of titrants and selecting suitable indicators can minimize these errors, while consistent stirring and correct sample preparation improve precision. Troubleshooting includes double-checking reagent concentrations, performing blank titrations, and using potentiometric methods when visual endpoints are unclear.
Practical Examples and Real-World Applications
Redox titration is frequently used to determine the concentration of oxidizing agents like potassium permanganate in water treatment, while back-titration is preferred for analyzing compounds that react slowly or incompletely, such as antacid formulations containing calcium carbonate. In environmental monitoring, redox titration helps quantify pollutants through oxidation-reduction reactions, whereas back-titration accurately measures residual reagents by adding an excess and titrating the unreacted portion, enhancing precision in pharmaceutical quality control. Industrial synthesis often relies on redox titrations for reaction endpoint detection, and back-titration ensures accurate assay of substances prone to side reactions or with insoluble products.
Redox Titration Infographic
 libterm.com
libterm.com