Base materials provide the essential foundation for construction, manufacturing, and engineering projects, ensuring stability and durability in various applications. Choosing the right base can significantly impact the performance and longevity of your structure or product. Discover how to select the ideal base material for your needs by reading the full article.
Table of Comparison
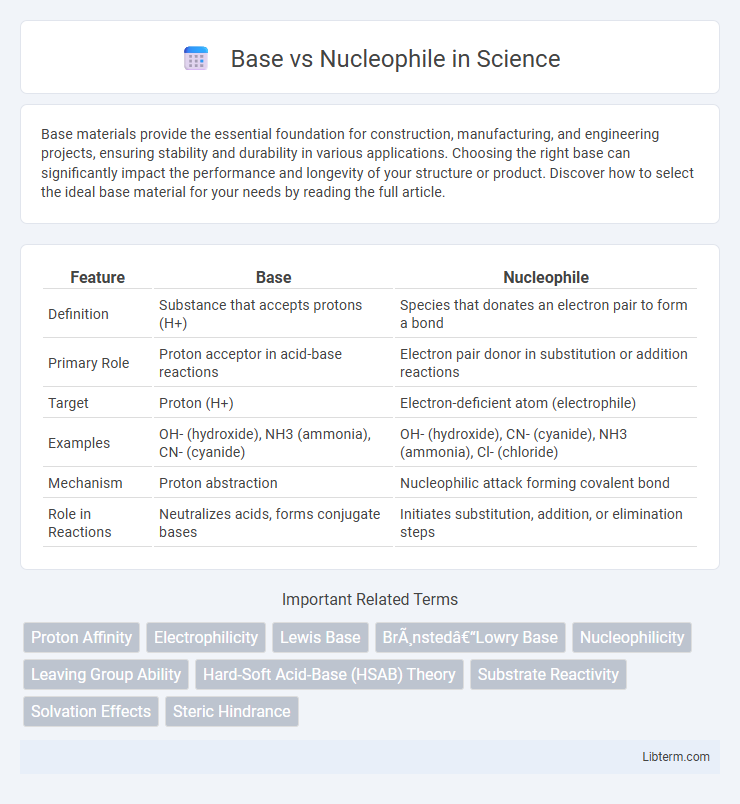
| Feature | Base | Nucleophile |
|---|---|---|
| Definition | Substance that accepts protons (H+) | Species that donates an electron pair to form a bond |
| Primary Role | Proton acceptor in acid-base reactions | Electron pair donor in substitution or addition reactions |
| Target | Proton (H+) | Electron-deficient atom (electrophile) |
| Examples | OH- (hydroxide), NH3 (ammonia), CN- (cyanide) | OH- (hydroxide), CN- (cyanide), NH3 (ammonia), Cl- (chloride) |
| Mechanism | Proton abstraction | Nucleophilic attack forming covalent bond |
| Role in Reactions | Neutralizes acids, forms conjugate bases | Initiates substitution, addition, or elimination steps |
Understanding the Concepts: Base and Nucleophile
A base is a substance that donates an electron pair to accept a proton, primarily involved in acid-base reactions, while a nucleophile donates an electron pair to form a bond with an electrophile in substitution or addition reactions. Bases typically target hydrogen ions (protons), whereas nucleophiles attack positively charged or electron-deficient atoms such as carbons. Understanding the difference is crucial for predicting reaction mechanisms and outcomes in organic chemistry.
Chemical Definitions: What is a Base? What is a Nucleophile?
A base is a chemical species that donates a pair of electrons to accept a proton, often defined by the Bronsted-Lowry theory as a proton acceptor, or by the Lewis theory as an electron pair donor. A nucleophile is an electron-rich atom or molecule that donates a pair of electrons to an electrophile, forming a new chemical bond during substitution or addition reactions. While all nucleophiles are bases in the Lewis sense, nucleophiles specifically target positively charged or electron-deficient centers, whereas bases primarily neutralize acids by accepting protons.
Structural Differences Between Bases and Nucleophiles
Bases and nucleophiles both donate electron pairs but differ structurally: bases generally have lone pairs localized on atoms with high electronegativity, favoring proton abstraction, while nucleophiles possess more accessible or polarizable lone pairs or p-electrons enabling attack on electrophilic carbon centers. Structural factors such as atom size, charge distribution, and orbital hybridization influence nucleophilicity, often enhanced by larger, more polarizable atoms compared to bases. Functional groups like amines and alkoxides exemplify these differences, with amines acting as stronger nucleophiles due to their less tightly held lone pairs compared to bases like hydroxides that prefer proton removal.
Electronic Properties: How Charge and Electronegativity Influence Reactivity
Bases typically possess a lone pair of electrons that can be donated, and their reactivity is influenced by charge density and electronegativity; negatively charged species tend to be stronger bases due to higher electron availability. Nucleophiles rely on electron-rich centers to attack electrophilic sites, where lower electronegativity atoms bear higher electron density, enhancing nucleophilicity. Charge stabilization and electronegativity differences critically determine the balance between nucleophilicity and basicity in chemical reactions.
Reaction Mechanisms: Roles of Bases vs. Nucleophiles
Bases primarily function by abstracting protons during reaction mechanisms, thereby increasing the electron density on adjacent atoms and facilitating the formation of reaction intermediates. Nucleophiles, on the other hand, donate electron pairs to electrophilic centers, leading to bond formation in substitution or addition reactions. The distinct roles of bases and nucleophiles critically influence mechanistic pathways such as elimination reactions (E2, E1) predominantly driven by bases, and nucleophilic substitution reactions (SN1, SN2) controlled by nucleophiles.
Examples of Common Bases and Nucleophiles in Organic Chemistry
Common bases in organic chemistry include hydroxide ion (OH-), alkoxide ions (RO-), and amines (RNH2), which primarily function by abstracting protons from acids. Nucleophiles such as halide ions (Cl-, Br-, I-), cyanide ion (CN-), and thiolate ions (RS-) donate electron pairs to electrophilic centers to form new covalent bonds. Understanding the distinct roles of bases and nucleophiles is critical for predicting reaction mechanisms like substitution and elimination.
Factors Affecting Strength: Solvent, Size, and Substituents
The strength of a base versus a nucleophile is significantly influenced by the solvent, with protic solvents stabilizing nucleophiles through hydrogen bonding, thereby reducing their nucleophilicity but often increasing basicity. Larger atoms or ions exhibit decreased nucleophilicity due to steric hindrance but may show enhanced basicity depending on charge density and orbital overlap. Substituents with electron-donating groups increase nucleophilicity by enhancing electron availability, while electron-withdrawing groups stabilize bases through resonance or inductive effects, altering both base strength and nucleophilicity.
Base vs. Nucleophile: Real-World Applications
Bases play a crucial role in industrial processes such as catalyzing the synthesis of pharmaceuticals by deprotonating reactants, while nucleophiles are essential in organic chemistry for forming carbon-carbon bonds through nucleophilic substitution reactions. In drug design, bases are used to adjust pH and facilitate reactions, whereas nucleophiles target electrophilic centers to create complex molecular structures. Understanding the distinct behavior of bases and nucleophiles enables chemists to optimize reaction pathways for efficient production of materials and active pharmaceutical ingredients.
Distinguishing Base Strength from Nucleophilicity
Base strength is determined by a species' ability to accept protons, directly related to its conjugate acid's pKa value, whereas nucleophilicity measures a species' ability to donate an electron pair to an electrophile during bond formation. Strong bases are often strong nucleophiles, but factors like solvent, steric hindrance, and charge influence nucleophilicity more significantly than basicity. For example, bulky bases like tert-butoxide exhibit high basicity but reduced nucleophilicity due to steric hindrance, highlighting the crucial distinction in reaction pathways and selectivity.
Key Takeaways: How to Identify and Choose Bases and Nucleophiles
Bases donate electron pairs to accept protons, while nucleophiles donate electron pairs to form bonds with electrophiles; identifying bases involves examining proton affinity and strength, whereas nucleophiles are identified by their polarizability and charge density. Strong bases often have high pKa conjugate acids and prefer deprotonation reactions, whereas strong nucleophiles exhibit high electron density and prefer substitution or addition reactions. Choosing between a base and a nucleophile depends on the reaction conditions, substrate structure, and desired product outcome.
Base Infographic
 libterm.com
libterm.com