Irreversible processes are those that cannot return to their original state without external intervention, often resulting in permanent changes. Understanding the nature of irreversibility is crucial in fields like thermodynamics, physics, and even everyday decision-making. Explore the article to uncover how irreversibility impacts your choices and the systems around you.
Table of Comparison
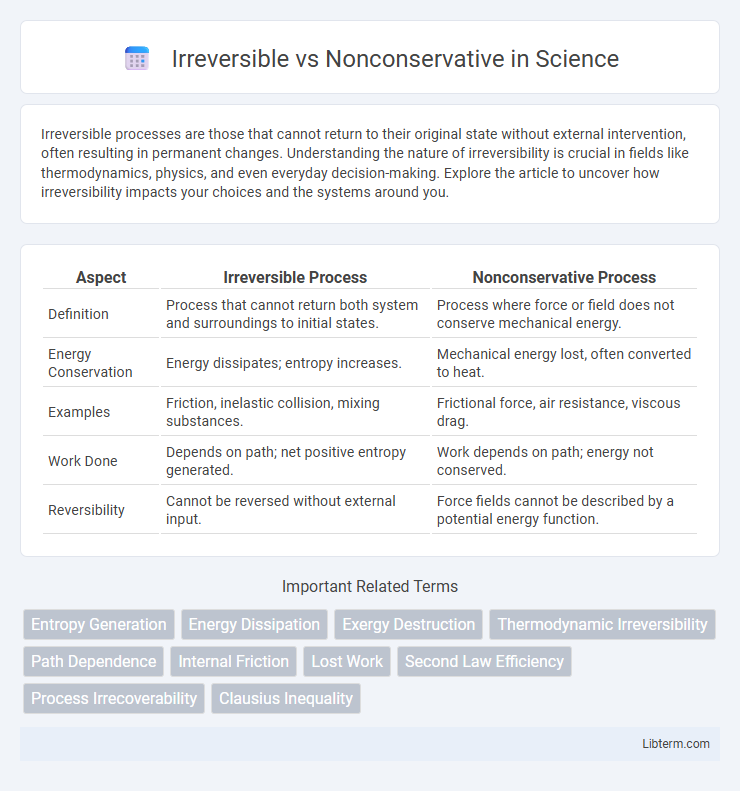
| Aspect | Irreversible Process | Nonconservative Process |
|---|---|---|
| Definition | Process that cannot return both system and surroundings to initial states. | Process where force or field does not conserve mechanical energy. |
| Energy Conservation | Energy dissipates; entropy increases. | Mechanical energy lost, often converted to heat. |
| Examples | Friction, inelastic collision, mixing substances. | Frictional force, air resistance, viscous drag. |
| Work Done | Depends on path; net positive entropy generated. | Work depends on path; energy not conserved. |
| Reversibility | Cannot be reversed without external input. | Force fields cannot be described by a potential energy function. |
Understanding Irreversible Processes
Irreversible processes are characterized by a deviation from thermodynamic equilibrium, causing entropy to increase and preventing the system from returning to its original state without external intervention. These processes involve factors such as friction, unrestrained expansion, heat transfer across finite temperature differences, and spontaneous chemical reactions, leading to energy dissipation. Nonconservative forces, like friction and viscous drag, contribute to irreversibility by converting useful energy into unusable forms, highlighting the fundamental difference between irreversible and reversible thermodynamic behavior.
Defining Nonconservative Systems
Nonconservative systems are characterized by forces that cause energy to dissipate, such as friction, air resistance, or inelastic deformation, preventing the total mechanical energy from remaining constant. Unlike conservative systems, where potential energy is fully recoverable and path-independent, nonconservative forces lead to energy loss primarily as heat or sound, making the system irreversible. These irreversible processes prevent the restoration of the original state without external energy input, defining the fundamental difference between nonconservative and reversible conservative systems.
Key Differences: Irreversible vs Nonconservative
Irreversible processes are thermodynamic changes where the system and surroundings cannot return to their original states without net energy loss, whereas nonconservative forces cause energy dissipation within mechanical systems, leading to irreversible energy transfer. Irreversibility is characterized by entropy generation, often found in natural phenomena such as friction, heat transfer, and mixing, while nonconservative forces refer specifically to forces like friction and air resistance that do work not conserved in mechanical energy. Understanding the distinction helps in analyzing energy conservation and efficiency in physical and engineering systems.
Examples of Irreversible Processes
Irreversible processes include natural phenomena such as mixing of gases, spontaneous heat transfer from hot to cold bodies, and unrestrained expansion of a gas into a vacuum. These processes result in an increase in entropy and cannot be reversed without external intervention. Nonconservative forces like friction and inelastic collisions also produce irreversible changes by dissipating mechanical energy into heat.
Real-World Nonconservative Forces
Nonconservative forces, such as friction, air resistance, and viscous drag, cause energy dissipation in the form of heat or sound, leading to irreversible processes in physical systems. These forces do not conserve mechanical energy because work done against them depends on the path taken, unlike conservative forces where energy is fully recoverable. Real-world applications often involve nonconservative forces, making it essential to account for energy losses when analyzing mechanical systems and thermodynamic cycles.
Impact on Energy Conservation
Irreversible processes cause a loss of useful energy due to entropy generation, resulting in a decrease in the total available energy for work, which violates the principle of energy conservation in practical applications. Nonconservative forces, such as friction and air resistance, convert mechanical energy into thermal energy, leading to energy dissipation within the system and reducing mechanical energy conservation. Understanding these distinctions is critical for accurately analyzing energy efficiency and optimizing thermodynamic system performance.
Thermodynamic Implications
Irreversible processes increase the entropy of a system and its surroundings, reflecting the generation of thermodynamic irreversibility and the loss of useful work potential. Nonconservative forces, such as friction and viscous dissipation, are primary contributors to these irreversible changes, converting mechanical energy into internal energy and driving entropy production. Understanding the thermodynamic implications of irreversible versus nonconservative phenomena is essential for optimizing energy efficiency and designing sustainable systems.
Reversibility in Physical Systems
Irreversible processes in physical systems lead to an increase in entropy, preventing the system from returning to its original state without external work, whereas nonconservative forces, like friction, dissipate energy as heat, making reversibility impossible. Reversibility requires idealized conditions with no entropy generation, ensuring all transformations can be undone without net energy loss or degradation. Understanding the distinction between irreversible and nonconservative effects is crucial for analyzing thermodynamic cycles and optimizing energy efficiency in engineering applications.
Practical Applications and Cases
Irreversible processes, such as real-world heat engines and chemical reactions, often lead to energy dissipation and increased entropy, impacting efficiency and system design in thermodynamics. Nonconservative forces, including friction and air resistance, cause energy loss in mechanical systems, necessitating compensation through external work in applications like vehicle dynamics and robotics. Understanding the distinction helps engineers optimize systems by minimizing energy loss and improving performance in practical scenarios like power plants and automated manufacturing.
Summary: Choosing the Right Concept
Irreversible processes involve entropy generation and energy dissipation, contrasting with nonconservative forces that cause energy loss within mechanical systems. Selecting the appropriate concept depends on the system's nature: irreversibility applies broadly to thermodynamic processes, while nonconservative forces relate to mechanical energy transformations. Understanding entropy production and energy conservation is crucial for accurate modeling and analysis in engineering and physics.
Irreversible Infographic
 libterm.com
libterm.com